Etonogestrel
Etonogestrel is a progestin medication which is used as a means of birth control for women.[2][3][6][7] It is available alone as an implant placed under the skin of the upper arm under the brand names Nexplanon and Implanon and in combination with ethinylestradiol, an estrogen, as a vaginal ring under the brand names NuvaRing and Circlet.[8] Etonogestrel is effective as a means of birth control within 8 hours of insertion.[9]
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Circlet, Implanon, Nexplanon, others |
| Other names | ORG-3236; SCH-900702 (with EE); 3-Ketodesogestrel; 3-Oxodesogestrel; 11-Methylenelevonorgestrel;[1] 11-Methylene-17α-ethynyl-18-methyl-19-nortestosterone; 11-Methylene-17α-ethynyl-18-methylestr-4-en-17β-ol-3-one |
| AHFS/Drugs.com | Professional Drug Facts |
| MedlinePlus | a604032 |
| Pregnancy category |
|
| Routes of administration | Subcutaneous implant, vaginal ring |
| Drug class | Progestogen; Progestin |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | Implant: 100%[2] Vaginal ring: 100%[3] |
| Protein binding | ≥98% (66% to albumin, 32% to SHBG)[2] |
| Metabolism | Liver (CYP3A4)[2][3] |
| Elimination half-life | 21–38 hours[4][5][2][3] |
| Excretion | Urine (major), feces (minor)[2][3] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.053.561 |
| Chemical and physical data | |
| Formula | C22H28O2 |
| Molar mass | 324.464 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Side effects of etonogestrel include menstrual irregularities, breast tenderness, mood changes, acne, headaches, vaginitis, and others.[2] Etonogestrel is a progestin, or a synthetic progestogen, and hence is an agonist of the progesterone receptor, the biological target of progestogens like progesterone.[10] It has very weak androgenic and glucocorticoid activity and no other important hormonal activity.[10]
Etonogestrel was patented in 1972 and introduced for medical use in 1998.[11][12][13] It became available in the United States in 2006.[11][12] Etonogestrel is sometimes referred to as a "third-generation" progestin.[14] It is marketed throughout the world.[8] A closely related and more widely known and used progestin, desogestrel, is a prodrug of etonogestrel in the body.[10]
Medical uses
Etonogestrel is used in hormonal contraception, most notably the etonogestrel contraceptive implant (brand names Nexplanon, Implanon) and the contraceptive vaginal ring (brand names NuvaRing, Circlet).[2][3]
Contraindications
Side effects
The most common side effects of etonogestrel when used as an implant, experienced by greater than or equal to 10% of women, include menstrual irregularities (with menstrual bleeding patterns including hypomenorrhea (33.6%), amenorrhea (22.2%), menorrhagia (17.7%), and polymenorrhea (6.7%)), headache (24.9%), vaginitis (14.5%), weight gain (13.7%), acne (13.5%), breast pain (12.8%), abdominal pain (10.9%), and pharyngitis (10.5%).[2] Less common side effects of etonogestrel when used as an implant, experienced by 5 to 10% of women, include leukorrhea (9.6%), implant site reactions (8.6%), influenza-like symptoms (7.6%), dizziness (7.2%), dysmenorrhea (7.2%), back pain (6.8%), emotional lability (6.5%), nausea (6.4%), pain (5.6%), nervousness (5.6%), depression (5.5%), hypersensitivity (5.4%), and insertion site pain (5.2%).[2] Implant site reactions included erythema (3.3%), hematoma (3.0%), bruising (2.0%), pain (1.0%), and swelling (0.7%).[2] Reasons for discontinuation of etonogestrel implant treatment included menstrual irregularities (11.1%), emotional lability (2.3–6.1%), weight gain (2.3%), headache (1.6%), acne (1.3%), and depression (1.0–2.4%).[2]
Other potential side effects of etonogestrel may include ectopic pregnancy, thrombosis and other vascular events, ovarian cysts, breast cancer, cervical cancer, cervical intraepithelial neoplasia, liver adenomas, elevated blood pressure, gallbladder disease, mild insulin resistance, small changes in glucose levels, hyperlipidemia, fluid retention, and visual changes or changes in lens tolerance in those with contact lenses.[2] Many additional possible side effects of etonogestrel have been reported in postmarketing surveillance.[2]
Etonogestrel is the active metabolite of the inactive prodrug desogestrel, one of two third-generation progestins found in some epidemiological studies of combined birth control pills to be associated with a higher risk of venous thrombosis than combined birth control pills containing certain second-generation progestins. Because hormones are released continuously from etonogestrel-containing vaginal rings, peak and total estrogen and progestin doses are significantly lower than with combined birth control pills, although it is not known whether this lowers the risk of blood clots.
Overdose
Interactions
Pharmacology
Pharmacodynamics
Etonogestrel is a progestogen, or an agonist of the progesterone receptor.[10] It is less androgenic than levonorgestrel and norethisterone,[15][16] and it does not cause a decrease in sex hormone-binding globulin levels.[17] However, it is still associated with acne in up to 13.5% of patients when used as an implant, though this side effect only accounts for 1.3% of premature removals of the implant.[9] In addition to its progestogenic and weak androgenic activity, etonogestrel binds to the glucocorticoid receptor with about 14% of the affinity of dexamethasone (relative to 1% for levonorgestrel) and has very weak glucocorticoid activity.[10] Etonogestrel has no other hormonal activity (e.g., estrogenic, antimineralocorticoid).[10] Some inhibition of 5α-reductase and hepatic cytochrome P450 enzymes has been observed with etonogestrel in vitro, similarly to other 19-nortestosterone progestins.[10]
| Compound | PR | AR | ER | GR | MR | SHBG | CBG |
|---|---|---|---|---|---|---|---|
| Etonogestrel | 150 | 20 | 0 | 14 | 0 | 15 | 0 |
| 5α-Dihydroetonogestrel | 9 | 17 | 0 | ? | ? | ? | ? |
| Sources: Values are percentages (%). Reference ligands (100%) were prome-gestone for the PR, metribolone for the AR, E2 for the ER, DEXA for the GR, aldosterone for the MR, DHT for SHBG, and cortisol for CBG. Sources: [18][10] | |||||||
| Steroid | Class | TR (↑)a | GR (%)b |
|---|---|---|---|
| Dexamethasone | Corticosteroid | ++ | 100 |
| Ethinylestradiol | Estrogen | – | 0 |
| Etonogestrel | Progestin | + | 14 |
| Gestodene | Progestin | + | 27 |
| Levonorgestrel | Progestin | – | 1 |
| Medroxyprogesterone acetate | Progestin | + | 29 |
| Norethisterone | Progestin | – | 0 |
| Norgestimate | Progestin | – | 1 |
| Progesterone | Progestogen | + | 10 |
| Footnotes: a = Thrombin receptor (TR) upregulation (↑) in vascular smooth muscle cells (VSMCs). b = RBA (%) for the glucocorticoid receptor (GR). Strength: – = No effect. + = Pronounced effect. ++ = Strong effect. Sources: [19] | |||
Pharmacokinetics
The bioavailability of etonogestrel when given as a subcutaneous implant or as a vaginal ring is 100%.[2][3] Steady-state levels of etonogestrel are achieved within one week upon insertion as an implant or vaginal ring.[2][3] The mean volume of distribution of etonogestrel is 201 L.[2] The plasma protein binding of the medication is at least 98%, with 66% bound to albumin and 32% bound to sex hormone-binding globulin.[2][3] Etonogestrel is metabolized in the liver by CYP3A4.[2][3] The biological activity of its metabolites is unknown.[2][3] The elimination half-life of etonogestrel is about 25 to 29 hours.[2][3] Following removal of an etonogestrel-containing implant, levels of the medication were below the limits of assay detection by one week.[2] The major portion of etonogestrel is eliminated in urine and a minor portion is eliminated in feces.[2][3]
Chemistry
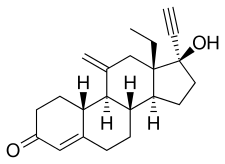
Etonogestrel, also known as 11-methylene-17α-ethynyl-18-methyl-19-nortestosterone or as 11-methylene-17α-ethynyl-18-methylestr-4-en-17β-ol-3-one, is a synthetic estrane steroid and a derivative of testosterone.[6][8] It is more specifically a derivative of norethisterone (17α-ethynyl-19-nortestosterone) and is a member of the gonane (18-methylestrane) subgroup of the 19-nortestosterone family of progestins.[20][21] Etonogestrel is the C3 ketone derivative of desogestrel and the C11 methylene derivative of levonorgestrel and is also known as 3-ketodesogestrel and as 11-methylenelevonorgestrel.[1]
History
Desogestrel (3-deketoetonogestrel), a prodrug of etonogestrel, was introduced for medical use in 1981.[4][22] Etonogestrel itself was first introduced, as Implanon in Indonesia, in 1998,[11][12] and was subsequently marketed in the United Kingdom shortly thereafter[23] and in the United States in 2006.[11][12]
Society and culture
Generic names
Etonogestrel is the generic name of the drug and its INN, USAN, and BAN.[6][8] It is also known by its developmental code name ORG-3236.[6][8]
Brand names
Etonogestrel is marketed under the brand names Circlet, Implanon, Nexplanon, and NuvaRing.[6][8]
Availability
Etonogestrel is available widely throughout the world, including in the United States, Canada, the United Kingdom, Ireland, elsewhere throughout Europe, South Africa, Latin America, South, East, and Southeast Asia, and elsewhere in the world.[8]
Research
An etonogestrel-releasing intrauterine device was under development for use as a form of birth control for women but development was discontinued in 2015.[24]
Etonogestrel has been studied for use as a potential male contraceptive.[25]
See also
References
- Kenneth J. Ryan (1999). Kistner's Gynecology and Women's Health. Mosby. p. 300. ISBN 978-0-323-00201-1.
- "Nexplanon- etonogestrel implant". DailyMed. 18 November 2019. Retrieved 25 September 2020.
- "NuvaRing- etonogestrel and ethinyl estradiol insert, extended release". DailyMed. 24 January 2020. Retrieved 25 September 2020.
- Benno Clemens Runnebaum; Thomas Rabe; Ludwig Kiesel (6 December 2012). Female Contraception: Update and Trends. Springer Science & Business Media. pp. 156–163. ISBN 978-3-642-73790-9.
- Mosby's GenRx: A Comprehensive Reference for Generic and Brand Prescription Drugs. Mosby. 2001. p. 687. ISBN 978-0-323-00629-3.
The elimination half-life for 3-keto-desogestrel is approximately 38 ± 20 hours at steady state.
- Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. p. 420. ISBN 978-3-88763-075-1.
- Thomas L. Lemke; David A. Williams (24 January 2012). Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. pp. 1409–. ISBN 978-1-60913-345-0.
- "Etonogestrel".
- Gretchen M Lentz; Rogerio A. Lobo; David M Gershenson; Vern L. Katz (21 February 2012). Comprehensive Gynecology. Elsevier Health Sciences. pp. 256–. ISBN 978-0-323-09131-2.
- Kuhl H (2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration" (PDF). Climacteric. 8 Suppl 1: 3–63. doi:10.1080/13697130500148875. PMID 16112947. S2CID 24616324.
- Helen Carcio; R. Mimi Secor (10 October 2014). Advanced Health Assessment of Women, Third Edition: Clinical Skills and Procedures. Springer Publishing Company. pp. 411–. ISBN 978-0-8261-2308-4.
- E. J. Mayeaux (28 March 2012). The Essential Guide to Primary Care Procedures. Lippincott Williams & Wilkins. pp. 589–. ISBN 978-1-4511-5286-9.
- Fischer, Jnos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 480. ISBN 9783527607495.
- Diana Vaamonde; Stefan S du Plessis; Ashok Agarwal (7 March 2016). Exercise and Human Reproduction: Induced Fertility Disorders and Possible Therapies. Springer. pp. 288–. ISBN 978-1-4939-3402-7.
- F. William Danby (27 January 2015). Acne: Causes and Practical Management. John Wiley & Sons. pp. 77–. ISBN 978-1-118-23277-4.
- David E. Golan (2008). Principles of Pharmacology: The Pathophysiologic Basis of Drug Therapy. Lippincott Williams & Wilkins. pp. 521–. ISBN 978-0-7817-8355-2.
- Leon Speroff; Philip D. Darney (22 November 2010). A Clinical Guide for Contraception. Lippincott Williams & Wilkins. pp. 365–. ISBN 978-1-60831-610-6.
- Kuhl H (1990). "Pharmacokinetics of oestrogens and progestogens". Maturitas. 12 (3): 171–97. doi:10.1016/0378-5122(90)90003-o. PMID 2170822.
- Kuhl H (2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration" (PDF). Climacteric. 8 Suppl 1: 3–63. doi:10.1080/13697130500148875. PMID 16112947. S2CID 24616324.
- Mary C. Brucker; Tekoa L. King (8 September 2015). Pharmacology for Women's Health. Jones & Bartlett Publishers. pp. 368–. ISBN 978-1-284-05748-5.
- Donna Shoupe (7 November 2007). The Handbook of Contraception: A Guide for Practical Management. Springer Science & Business Media. pp. 16–. ISBN 978-1-59745-150-5.
- Jeremy A. Holtsclaw (2007). Progress Towards the Total Synthesis of Desogestrel and the Development of a New Chiral Dihydroimidazol-2-ylidene Ligand. University of Michigan. p. 25.
In 1981, desogestrel was marketed as a new low dose oral contraceptive under the trade names Marvelon® and Desogen®.32
- Anna Glasier; Beverly Winikoff (December 1999). Contraception. Health Press. p. 41. ISBN 978-1-899541-18-8.
- "Etonogestrel-releasing intrauterine system - Merck & Co. - AdisInsight".
- Nieschlag E (2010). "Clinical trials in male hormonal contraception" (PDF). Contraception. 82 (5): 457–70. doi:10.1016/j.contraception.2010.03.020. PMID 20933120.
Further reading
- Bennink HJ (September 2000). "The pharmacokinetics and pharmacodynamics of Implanon, a single-rod etonogestrel contraceptive implant". Eur J Contracept Reprod Health Care. 5 Suppl 2: 12–20. PMID 11246602.
- Varma R, Mascarenhas L (June 2001). "Endometrial effects of etonogestrel (Implanon) contraceptive implant". Curr. Opin. Obstet. Gynecol. 13 (3): 335–41. doi:10.1097/00001703-200106000-00015. PMID 11396660. S2CID 45520124.
- "Etonogestrel implant (Implanon) for contraception". Drug Ther Bull. 39 (8): 57–9. August 2001. PMID 11526801.
- Gaffield ME, Curtis KM, Mohllajee AP, Peterson HB (February 2006). "Medical eligibility criteria for new contraceptive methods: combined hormonal patch, combined hormonal vaginal ring and the etonogestrel implant". Contraception. 73 (2): 134–44. doi:10.1016/j.contraception.2005.08.002. PMID 16413844.
- Wagner MS, Arias RD, Nucatola DL (August 2007). "The combined etonogestrel/ethinyl estradiol contraceptive vaginal ring". Expert Opin Pharmacother. 8 (11): 1769–77. doi:10.1517/14656566.8.11.1769. PMID 17685892. S2CID 42412888.
- Mansour D, Bahamondes L, Critchley H, Darney P, Fraser IS (March 2011). "The management of unacceptable bleeding patterns in etonogestrel-releasing contraceptive implant users". Contraception. 83 (3): 202–10. doi:10.1016/j.contraception.2010.08.001. PMID 21310280.
- "In brief: etonogestrel (nexplanon) contraceptive implant". Med Lett Drugs Ther. 54 (1383): 12. February 2012. PMID 22354222.
- Stuebe AM, Bryant AG, Lewis R, Muddana A (May 2016). "Association of Etonogestrel-Releasing Contraceptive Implant with Reduced Weight Gain in an Exclusively Breastfed Infant: Report and Literature Review". Breastfeed Med. 11 (4): 203–6. doi:10.1089/bfm.2016.0017. PMC 4860664. PMID 27032034.
- López-Picado A, Lapuente O, Lete I (April 2017). "Efficacy and side-effects profile of the ethinylestradiol and etonogestrel contraceptive vaginal ring: a systematic review and meta-analysis". Eur J Contracept Reprod Health Care. 22 (2): 131–146. doi:10.1080/13625187.2017.1287351. PMID 28256919. S2CID 24447820.
External links