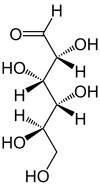
Fischer projection
The Fischer projection, devised by Emil Fischer in 1891,[1] is a two-dimensional representation of a three-dimensional organic molecule by projection. Fischer projections were originally proposed for the depiction of carbohydrates and used by chemists, particularly in organic chemistry and biochemistry. The use of Fischer projections in non-carbohydrates is discouraged, as such drawings are ambiguous when confused with other types of drawing.[2]
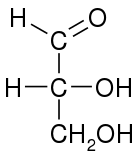
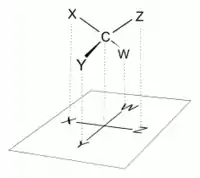

Conventions
All non-terminal bonds are depicted as horizontal or vertical lines. The carbon chain is depicted vertically, with carbon atoms sometimes not shown and represented by the center of crossing lines (see figure below). The orientation of the carbon chain is so that the first carbon (C1) is at the top.[3] In an aldose, C1 is the carbon of the aldehyde group; in a ketose, C1 is the carbon closest to the ketone group, which is typically found at C2.[4]
In a Fischer projection, all horizontal bonds are meant to be slanted toward the viewer. Molecules with a simple tetrahedral geometry can be easily rotated in space, so that this condition is met (see figures). For instance, a monosaccharide with three carbon atoms (triose), such as the D-Glyceraldehyde depicted above has a tetrahedral geometry, with C2 at its center, and can be rotated in space so that the carbon chain is vertical with C1 at the top, and the horizontal bonds connecting C2 with -H and -OH are both slanted toward the viewer.
However, when creating a Fischer projection for a monosaccharide with more than three carbons, there's no way to orient the molecule in space so that all horizontal bonds will be slanted toward the viewer. After rotating the molecule so that both the horizontal bonds with C2 are slanted toward the viewer, the horizontal bonds with C3 will be typically slanted away. So, after drawing the bonds with C2, before drawing the bonds with C3 the molecule must be rotated in space by 180° about its vertical axis. Further similar rotations may be needed to complete the drawing.
This implies that in most cases a Fischer projection is not an accurate representation of the actual 3D configuration of a molecule. It can be regarded as a projection of a modified version of the molecule, ideally twisted at multiple levels along its backbone. For instance, an open-chain molecule of D-glucose rotated so that the horizontal bonds with C2 are slanted toward the viewer, would have the bonds with C3 and C5 slanted away from the viewer, and hence its accurate projection would not coincide with a Fischer projection. For a more accurate representation of an open-chain molecule, a Natta projection may be used.
According to IUPAC rules, all hydrogen atoms should preferably be drawn explicitly; in particular, the hydrogen atoms of the end group of carbohydrates should be present. [2] In this regard Fischer projection is different from skeletal formulae.
Usage
Fischer projections are most commonly used in biochemistry and organic chemistry to represent monosaccharides. They can also be used for amino acids or for other organic molecules, although this is discouraged by the 2006 IUPAC recommendations.[2]
A Fischer projection can be used to differentiate between L- and D- molecules. For instance, by definition, in a Fischer projection the penultimate (next-to-last) carbon of D-sugars are depicted with hydrogen on the left and hydroxyl on the right. L-sugars will be shown with the hydrogen on the right and the hydroxyl on the left.[5]
Other systems
Haworth projections are a related chemical notation used to represent sugars in ring form. The groups on the right hand side of a Fischer projection are equivalent to those below the plane of the ring in Haworth projections.[6] Fischer projections should not be confused with Lewis structures, which do not contain any information about three dimensional geometry. Wedge-and-dash notation is used to represent the stereochemistry of most classes of organic compounds, with Newman projections being used to depict specific conformations of rotatable bonds of organic molecules (including but not limited to carbohydrates).
See also
| Wikimedia Commons has media related to Fischer projection. |
References
- John McMurry (2008). Organic Chemistry (7th ed.). Brooks/Cole - Thomson Learning, Inc. p. 975. ISBN 978-0-13-286261-5.
- Graphical representation of stereochemical configuration (IUPAC Recommendations 2006), p.1933-1934
- Understanding Fischer Projection and Angular Line Representation Conversion Luis F. Moreno Journal of Chemical Education 2012 89 (1), 175-176 doi:10.1021/ed101011c
- "Rules of Carbohydrate Nomenclature". The Journal of Organic Chemistry. 28 (2): 281–291. February 1963. doi:10.1021/jo01037a001.
- "Sugars & Polysaccharides". Rensselaer Polytechnic Institute (RPI). Archived from the original on 2011-07-12. Retrieved 2011-07-10.
- Matthews, C. E.; K. E. Van Holde; K. G. Ahern (1999) Biochemistry. 3rd edition. Benjamin Cummings. ISBN 0-8053-3066-6