Fluxional molecule
Fluxional (or non-rigid) molecules are molecules that undergo dynamics such that some or all of their atoms interchange between symmetry-equivalent positions. Because virtually all molecules are fluxional in some respects, e.g. bond rotations in most organic compounds, the term fluxional depends on the context and the method used to assess the dynamics. Often, a molecule is considered fluxional if its spectroscopic signature exhibits line-broadening (beyond that dictated by the Heisenberg uncertainty principle) due to chemical exchange. In some cases, where the rates are slow, fluxionality is not detected spectroscopically, but by isotopic labeling. Where such movement does not occur, the molecule may be described as a semi-rigid molecule.[1][2][3][4] Longuet-Higgins introduced the use of permutation-inversion groups for the symmetry classification of the states of fluxional (or non-rigid) molecules.[5][6]
A well-studied fluxional ion is the methanium ion, which is protonated methane, CH+
5.[7][8][9] In this unusual species, whose IR spectrum was recently experimentally observed[10][8] and more recently understood,[11][12][13] the barriers to proton exchange are lower than the zero-point energy. Thus, even at absolute zero there is no rigid molecular structure; the H atoms are always in motion. More precisely, the spatial distribution of protons in CH+
5 is many times broader than its parent molecule CH4, methane.[14][15]
Spectroscopic studies
NMR spectroscopy
Temperature dependent changes in the NMR spectra result from dynamics associated with the fluxional molecules when those dynamics proceed at rates comparable to the frequency differences observed by NMR. The experiment is called DNMR and typically involves recording spectra at various temperatures. In the ideal case, low temperature spectra can be assigned to the "slow exchange limit", whereas spectra recorded at higher temperatures correspond to molecules at "fast exchange limit". Typically, high temperature spectra are simpler than those recorded at low temperatures, since at high temperatures, equivalent sites are averaged out. Prior to the advent of DNMR, kinetics of reactions were measured on non-equilibrium mixtures, monitoring the approach to equilibrium.
Many molecular processes exhibit fluxionality that can be probed on the NMR time scale.[16] Beyond the examples highlighted below, other classic examples include the Cope rearrangement in bullvalene and the chair inversion in cyclohexane.
For processes that are too slow for traditional DNMR analysis, the technique spin saturation transfer (SST) is applicable. This magnetization transfer technique provides rate information, provided that the rates exceed 1/T1.[17]
Pentacoordinate coordination
The prototypical fluxional molecule is phosphorus pentafluoride. Its 19F NMR spectrum consists of a 31P-coupled doublet, indicating that the equatorial and axial fluorine centers interchange rapidly on the NMR timescale. Fluorine-19 NMR spectroscopy, even at temperatures as low as −100 °C, fails to distinguish the axial from the equatorial fluorine environments. The apparent equivalency arises from the low barrier for pseudorotation via the Berry mechanism, by which the axial and equatorial fluorine atoms rapidly exchange positions.[18]
Berry pseudorotation
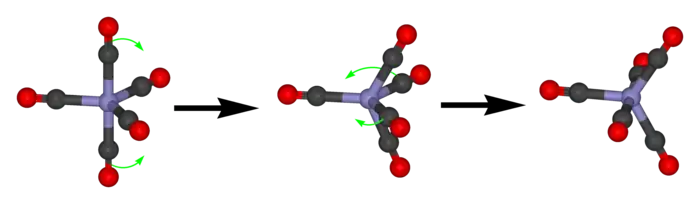
Pentacoordinate molecules of trigonal pyramidal geometry typically exhibit a particular kind of low energy fluxional behavior called Berry pseudorotation. Famous examples of such molecules are iron pentacarbonyl (Fe(CO)5) and phosphorus pentafluoride (PF5). At higher temperatures, only one signal is observed for the ligands (e.g., by 13C or 19F NMR) whereas at low temperatures, two signals in a 2:3 ratio can be resolved. Molecules that are not strictly pentacoordinate are also subject to this process, such as SF4.
Dimethylformamide
A classic example of a fluxional molecule is dimethylformamide.[19]

At temperatures near 100 °C, the 500 MHz NMR spectrum of this compound shows only one signal for the methyl groups. Near room temperature however, separate signals are seen for the non-equivalent methyl groups. The rate of exchange can be readily calculated at the temperature where the two signals are just merged. This "coalescence temperature" depends on the measuring field. The relevant equation is:
where Δνo is the difference in Hz between the frequencies of the exchanging sites. These frequencies are obtained from the limiting low-temperature NMR spectrum. At these lower temperatures, the dynamics continue, of course, but the contribution of the dynamics to line broadening is negligible.
For example, if Δνo = 1ppm @ 500 MHz
- (ca. 0.5 millisecond half-life)
Ring whizzing in organometallic chemistry
Many organometallic compounds exhibit fluxionality.[20] The compound Fe(η5-C5H5) (η1- C5H5)(CO)2 exhibits the phenomenon of "ring whizzing".
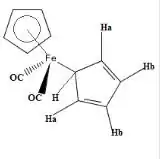
At 30 °C, the 1H NMR spectrum shows only two peaks, one typical (δ5.6) of the η5-C5H5 and the other assigned η1-C5H5. The singlet assigned to the η1-C5H5 ligand splits at low temperatures owing to the slow hopping of the Fe center from carbon to carbon in the η1-C5H5 ligand.[21] Two mechanisms have been proposed, with the consensus favoring the 1,2 shift pathway.[22]
IR spectroscopy
Although less common, some dynamics are also observable on the time-scale of IR spectroscopy. One example is electron transfer in a mixed-valence dimer of metal clusters. Application of the equation for coalescence of two signals separated by 10 cm−1 gives the following result:[23]
Clearly, processes that induce line-broadening on the IR time-scale must be extremely rapid.
Identical nuclei
When a molecule contains identical nuclei—which is commonly the case—there are a number of minima related by the permutations of the identical nuclei. The minima, distinguished by different numberings of identical nuclei, can be partitioned in equivalent classes. Two minima are equivalent if they can be transformed into one other by rotating the molecule, that is, without surmounting any energy barrier (bond breaking or bond twisting). The molecules with minima in different equivalent classes are called versions.[24] To transform one version into another version an energy barrier must be overcome.
Example
Take for instance the pyramidal ammonia (NH3) molecule. There are 3! = 6 permutations of the hydrogen atoms. If we count the hydrogen atoms looking down from the nitrogen atom onto the plane of the hydrogen atoms, then we see that
- {H1-H2-H3, H3-H1-H2, H2-H3-H1}
forms one equivalence class, (class I), because the members can be transformed into each other by simply rotating around the 3-fold axis without overcoming an energy barrier.
The other equivalence class (class II) consists of
- {H2-H1-H3, H3-H2-H1, H1-H3-H2}.
To transform a member (version) of class I to class II, an energy barrier has to be overcome. (The lowest path on the potential energy surface is actually via the flipping of the ammonia "umbrella". The umbrella up and the umbrella down are separated by an energy barrier of height of about 1000 cm−1).
In a semi-rigid molecule all the barriers between different versions are so high that tunneling though the barriers may be neglected. Under these conditions identical nuclei may be seen as distinguishable particles, to which the Pauli exclusion principle does not apply. This is a very common point of view in chemistry.
See also
References
- H. W. Kroto, Molecular Rotation Spectra, Wiley, New York, 1975 (Reprinted by Dover 1992)
- Philip R. Bunker and Per Jensen, Molecular Symmetry and Spectroscopy, 2nd edition, NRC Research Press, Ottawa, 1998
- D. Papoušek and M. R. Aliev, Molecular Vibrational-Rotational Spectra Elsevier, Amsterdam, 1982
- E. B. Wilson, J. C. Decius, and P. C. Cross, Molecular Vibrations, McGraw-Hill, New York, 1955 (Reprinted by Dover 1980)
- Longuet-Higgins, H.C. (1963). "The symmetry groups of non-rigid molecules". Molecular Physics. 6 (5): 445–460. Bibcode:1963MolPh...6..445L. doi:10.1080/00268976300100501.
- P. R. Bunker and P. Jensen, Fundamentals of Molecular Symmetry, CRC Press, 1998 ISBN 0-7503-0941-5
- Kramer, G. M. (1999). "CH5+ Stability and Mass Spectrometry". Science. 286 (5442): 1051a–1051. doi:10.1126/science.286.5442.1051a. ISSN 0036-8075.
- E. T. White; J. Tang; T. Oka (1999). "CH5+: The infrared spectrum observed". Science. 284 (5411): 135–7. Bibcode:1999Sci...284..135W. doi:10.1126/science.284.5411.135. PMID 10102811.
- Marx, D. (1999). "MOLECULAR SPECTROSCOPY:CH5+: The Cheshire Cat Smiles". Science. 284 (5411): 59–61. Bibcode:1999Sci...284...59.. doi:10.1126/science.284.5411.59. ISSN 0036-8075. S2CID 94058015.
- D. W. Boo; Z. F. Liu; A. G. Suits; J. S. Tse; Y. T. Lee (1995). "Dynamics of Carbonium Ions Solvated by Molecular Hydrogen: CH5+(H2)n (n= 1, 2, 3)". Science. 269 (5220): 57–9. Bibcode:1995Sci...269...57B. doi:10.1126/science.269.5220.57. PMID 17787703.
- Asvany, O.; Kumar P, P.; Redlich, B.; Hegemann, I.; Schlemmer, S.; Marx, D. (2005). "Understanding the Infrared Spectrum of Bare CH5+". Science. 309 (5738): 1219–1222. Bibcode:2005Sci...309.1219A. doi:10.1126/science.1113729. PMID 15994376.
- Xiao-Gang Wang; Tucker Carrington Jr (2016). "Calculated rotation-bending energy levels of CH5+ and a comparison with experiment". Journal of Chemical Physics. 144 (20): 204304. Bibcode:2016JChPh.144t4304W. doi:10.1063/1.4948549. PMID 27250303.
- H. Schmiedt; Per Jensen; S. Schlemmer (2017). "Rotation-vibration motion of extremely flexible molecules - The molecular superrotor". Chemical Physics Letters. 672: 34–46. Bibcode:2017CPL...672...34S. doi:10.1016/j.cplett.2017.01.045.
- Thompson, KC; Crittenden, DL; Jordan, MJ (2005). "CH5+: Chemistry's chameleon unmasked". J. Am. Chem. Soc. 127 (13): 4954–4958. doi:10.1021/ja0482280. PMID 15796561.
- For an animation of the dynamics of CH5+, see http://www.theochem.ruhr-uni-bochum.de/research/marx/topic4b.en.html Archived 2007-12-24 at the Wayback Machine
- Bryant, Robert G. (1983). "The NMR time scale". Journal of Chemical Education. 60 (11): 933. Bibcode:1983JChEd..60..933B. doi:10.1021/ed060p933.
- Jarek, R. L.; Flesher, R. J.; Shin, S. K. (1997). "Kinetics of Internal Rotation of N,N-Dimethylacetamide: A Spin-Saturation Transfer Experiment". Journal of Chemical Education. 74 (8): 978. doi:10.1021/ed074p978.
- Gutowsky, H. S.; McCall, D. W.; Slichter, C. P. (1953). "Nuclear Magnetic Resonance Multiplets in Liquids". J. Chem. Phys. 21 (2): 279. Bibcode:1953JChPh..21..279G. doi:10.1063/1.1698874.
- H. S. Gutowsky; C. H. Holm (1956). "Rate Processes and Nuclear Magnetic Resonance Spectra. II. Hindered Internal Rotation of Amides". J. Chem. Phys. 25 (6): 1228–1234. Bibcode:1956JChPh..25.1228G. doi:10.1063/1.1743184.
- John W. Faller "Stereochemical Nonrigidity of Organometallic Complexes" Encyclopedia of Inorganic and Bioinorganic Chemistry 2011, Wiley-VCH, Weinheim. doi:10.1002/9781119951438.eibc0211
- Bennett, Jr. M. J.; Cotton, F. A.; Davison, A.; Faller, J. W.; Lippard, S. J.; Morehouse, S. M. (1966). "Stereochemically Nonrigid Organometallic Compounds. I. π-Cyclopentadienyliron Dicarbonyl σ-Cyclopentadiene". J. Am. Chem. Soc. 1966 (88): 4371. doi:10.1021/ja00971a012.
- Robert B. Jordan, Reaction Mechanisms of Inorganic and Organometallic Systems (Topics in Inorganic Chemistry), 2007. ISBN 978-0195301007
- Casey H. Londergan; Clifford P. Kubiak (2003). "Electron Transfer and Dynamic Infrared-Band Coalescence: It Looks Like Dynamic NMR Spectroscopy, but a Billion Times Faster". Chemistry: A European Journal. 9 (24): 5969ff. doi:10.1002/chem.200305028. PMID 14679508.
- Bone, R.G.A.; et al. (1991). "Transition states from molecular symmetry groups: Analysis of non-rigid acetylene trimer". Molecular Physics. 72 (1): 33–73. doi:10.1080/00268979100100021.