Formic acid (data page)
This page provides supplementary chemical data on formic acid.
Material Safety Data Sheet
The handling of this chemical may incur notable safety precautions. It is highly recommend that you seek the Material Safety Datasheet (MSDS) for this chemical from a reliable source and follow its directions.
- MSDS from FLUKA in the SDSdata.org database
- Science Stuff
Structure and properties
| Structure and properties | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Index of refraction, nD | 1.3714 at 20 °C | ||||||||
| Abbe number | ? | ||||||||
| Dielectric constant, εr | 58.5 ε0 at 16 °C measured at 400 MHz | ||||||||
| Bond strength | ? | ||||||||
| Bond length[1] | C=O bond: 123 pm C–OH bond: 136 pm | ||||||||
| Bond angle | ? | ||||||||
| Magnetic susceptibility | ? | ||||||||
| Surface tension | 37.67 dyn/cm at 20 °C | ||||||||
| Viscosity[2] |
| ||||||||
Thermodynamic properties
| Phase behavior | |
|---|---|
| Triple point | 281.40 K (8.25 °C), 2.2 kPa |
| Critical point[3] | 588 K (315 °C), 5.81 MPa |
| Std enthalpy change of fusion, ΔfusH |
+12.68 kJ/mol |
| Std entropy change of fusion, ΔfusS |
+45.05 J/(mol·K) at 8.25 °C |
| Std enthalpy change of vaporization, ΔvapH |
+23.1 kJ/mol |
| Std entropy change of vaporization, ΔvapS |
+61.8 J/(mol·K) |
| Solid properties | |
| Std enthalpy change of formation, ΔfH |
? kJ/mol |
| Standard molar entropy, S |
? J/(mol K) |
| Heat capacity, cp | 74.5 J/(mol K) at –22 °C 82.8 J/(mol K) at 0 °C |
| Liquid properties | |
| Std enthalpy change of formation, ΔfH |
–425.5 kJ/mol |
| Standard molar entropy, S |
129.0 J/(mol K) |
| Enthalpy of combustion, ΔcH |
–254.6 kJ/mol |
| Heat capacity, cp | 101.3 J/(mol K) at 20–100 °C |
| Gas properties | |
| Std enthalpy change of formation, ΔfH |
–362.6 kJ/mol |
| Standard molar entropy, S |
251.0 J/(mol K) |
| Heat capacity, cp | 45.68 J/(mol K) at 25 °C |
Vapor pressure of liquid
| P in mm Hg | 1 | 10 | 40 | 100 | 400 | 760 | |
| T in °C | –20.0(s) | 2.1(s) | 24.0 | 43.8 | 80.3 | 100.6 | |
Table data obtained from CRC Handbook of Chemistry and Physics, 44th ed. The "(s)" notation indicates temperature of solid/vapor equilibrium. Otherwise the data is temperature of liquid/vapor equilibrium.
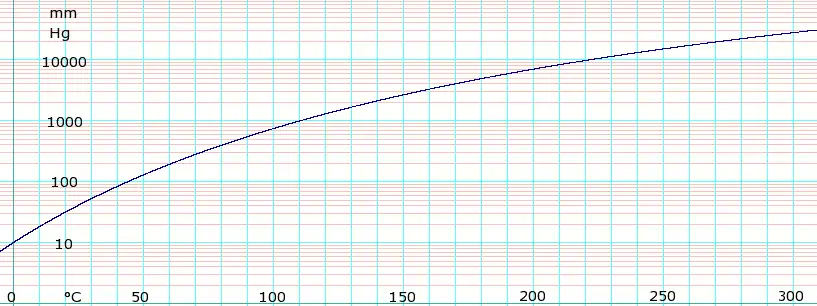
log10 of formic acid vapor pressure. Uses formula: obtained from Lange's Handbook of Chemistry, 10th ed.
Distillation data
| Vapor-liquid Equilibrium for Formic acid/Water[4] P = 760 mmHg | ||
| BP Temp. °C |
% by mole water | |
|---|---|---|
| liquid | vapor | |
| 102.3 | 4.05 | 2.45 |
| 104.6 | 15.5 | 10.2 |
| 105.9 | 21.8 | 16.2 |
| 107.1 | 32.1 | 27.9 |
| 107.6 | 40.9 | 40.2 |
| 107.6 | 41.1 | 40.5 |
| 107.6 | 46.4 | 48.2 |
| 107.1 | 52.2 | 56.7 |
| 106.0 | 63.2 | 71.8 |
| 104.2 | 74.0 | 83.6 |
| 102.9 | 82.9 | 91.7 |
| 101.8 | 90.0 | 95.1 |
Spectral data
| UV-Vis | |
|---|---|
| λmax | ? nm |
| Extinction coefficient, ε | ? |
| IR | |
| Major absorption bands | ? cm−1 |
| NMR | |
| Proton NMR | |
| Carbon-13 NMR | |
| Other NMR data | |
| MS | |
| Masses of main fragments |
|
Safety data
| Formic acid | |
|---|---|
| EINECS number | 200-579-1 |
| Safety data | |
| EU classification | Corrosive (C) |
| PEL | 5 ppm (9 mg/m3) |
| IDLH | 30 ppm |
| Autoignition temperature | 520 °C |
| Explosive limits | 18–51% |
References
- Morrison, Robert Thornton and Boyd, Robert Neilson, Organic Chemistry, 2nd ed. Allyn and Bacon 1972, p 596
- Lange's Handbook of Chemistry, 10th ed. pp 1669-1674
- Thornton Schnabel; Marfa Cortada; Jadran Vrabec; Santiago Lago; Hans Hasse. "Molecular Model for Formic Acid adusted to Vapor-Liquid Equilibrium" (PDF). Insitut für Technische Thermodynamik und Thermische Verfahrenstechnik (Institute for Technical Thermodynamic and Thermal Process Engineering), Universität Stuttgart. Archived from the original (PDF) on 12 June 2007. Retrieved 3 May 2007.
- "Binary Vapor-Liquid Equilibrium Data" (Queriable database). Chemical Engineering Research Information Center. Retrieved 6 May 2007.
- "Pure Component Properties". Chemical Information Research Information Center. Archived from the original on 2 August 2002. Retrieved 4 May 2007.
Except where noted otherwise, data relate to standard ambient temperature and pressure.
Disclaimer applies.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.