Freshwater acidification
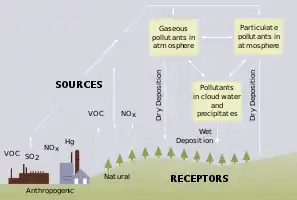
Freshwater becomes acidic when acid inputs surpass the quantity of bases produced in the reservoir through weathering of rocks, or by the reduction of acid anions, like sulfate and nitrate within the lake.[1] The main reason for freshwater acidification is atmospheric depositions and soil leaching of SOx and NOx.[1] In an acid-sensitive ecosystem, which includes slow-weathering bedrock and depleted base cation pools, SOx and NOx from runoff will be accompanied by acidifying hydrogen ions and inorganic aluminum, which can be toxic to marine organisms.[1] Acid rain is also a contributor to freshwater acidification, however acid rain is formed when SOx and NOx react with water, oxygen and oxidants within the clouds.[2] In addition to SOx and NOx, the buffering capacity of soils and bedrocks within the freshwater ecosystem can contribute to the acidity of the water. Each freshwater reservoir has a capacity to buffer acids.[1] However, with excess input of acids into the reservoir, the buffering capacity will essentially “run out” and the water will eventually become more acidic.[1] Increase in atmospheric CO2 affects freshwater acidity very similarly to the way rising CO2 affects ocean ecosystems.[3] However, because of the various carbon fluxes in freshwater ecosystems, it is difficult to quantify the effects of anthropogenic CO2.[4] Finally, rising freshwater acidification is harmful to various aquatic organisms.
Freshwater vs. Ocean Acidification
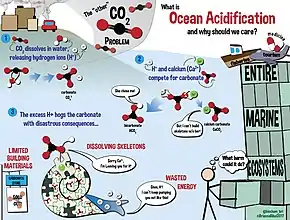
The ocean and the atmosphere are constantly exchanging massive amounts of CO2.[3] Over the last 800 000 years, the concentration of CO2 in the atmosphere remained around 172-300 parts per million by volume (ppmv).[3] However, with recent anthropogenic CO2 emissions, this number has increased to 387 ppmv in 2009.[3] From 2000-2008, 26% of anthropogenic CO2 was absorbed by the ocean.[3] Although ocean acidification is also caused by other chemical additions and removals, CO2 is the primary factor affecting pH.[3] Once CO2 is dissolved in seawater, it becomes a weak acid that primarily affects carbonate chemistry.[3] Dissolved CO2 increases the concentration of bicarbonate ions (HCO3−), dissolved inorganic carbon (CT) and lowers the pH.[3] Freshwater also absorbs atmospheric CO2, which can also lower the pH.[4] In addition to CO2, freshwater reservoir's pH values are altered by acid rain, nutrient runoff, and other anthropogenic pollutants.[4] Freshwater uptakes CO2 in the same mechanism as seawater, however, freshwater alkalinity is much lower than seawater, due to the absence of a salt-buffer.[4] Due to the lack of salt-buffer, pH changes in freshwater tend to be much greater than ocean water, due to newly released H+ ions not being buffered by as many bicarbonate (HCO3−) ions as ocean water.[4] Therefore, the freshwater biota tends to have a higher evolutionary pH tolerance than seawater biota.[4]
Causes
SOx and NOx
The accelerated burning of fossil fuels within the past century has largely contributed to the acidification of freshwater ecosystems. In the 1970s, the sulfate emissions levels peaked, with nitrogen following behind 10 years later.[5] The main contributors to freshwater acidification are SOx and NOx. Increases in sulfate concentration in runoff, due to increased acidity inputs, is coupled with an increase in base cation run-off and bicarbonate decrease, creating the acidifying effect seen in freshwater ecosystems.[6] In a natural state, most nitrogen inputted into freshwater ecosystems will be utilized by vegetation.[6] However, in excess amounts, all of the nitrogen is unable to be utilized by vegetation, and excess nitrogen is found as nitrate in the water's runoff.[6] Nitrate will contribute to acidification in the same manner as sulfate.[6]
Buffering Capacity
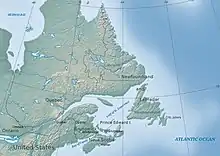
In addition to SOx and NOx, low buffering capacities of ecosystems can also lead to freshwater acidity. For example, Atlantic Canada has the lowest acid deposition rates in Eastern North America, with the most acidic waters on the continent.[7] This is due to the low buffering of the regional bedrock and the addition of natural organic acids produced by close by wetlands.[7] Specifically, in Southwestern and Eastern Nova Scotia, there is a combination of high organic acidity, poor buffering, and high acid deposition to produce a very low surface water pH and acid neutralization capacity (ANC) values.[7] In most of the Atlantic region, granite and shale bedrock are found, which contain very little buffering material.[7] Soil formed from low-buffering materials and the waters that drain from them are, therefore, susceptible to acidification, even under low acid deposition.[7]
CO2
In oceans, CO2 in the atmosphere can dissolve into the water's surface, and forms carbonic acid.[8] The total inorganic carbon in freshwater involves free CO2 (or H2CO3), HCO3− and carbonate (CO32-).[9] The percentage of all these constituents is also dependent on the pH of the body of water.[9] When water is acidic it will primarily contain CO2.[9] It is often difficult to quantify the effects of pCO2 levels in freshwater due to the various sources of carbon dioxide freshwater ecosystems receive. Factors such as nearby ecosystem, agriculture, land use, watershed, lake size, precipitation, soil type and rocks all determine the amount of CO2 absorbed.[8] However, there has been a clear increase in pCO2 in freshwater ecosystems in the last century due to anthropogenic influence.[8] As the vegetation near freshwater ecosystems grow larger and multiply, due to the excess pCO2 feeding these plants, the carbon available at death and during decomposition increases.[8] Then, precipitation, weathering and runoff will wash this soil into the nearby water.[8] When the pCO2 from the decomposing vegetation reacts with the water, it forms carbonic acid, which contributes to a lower pH level.
Harmful Effects on Aquatic Ecosystems
%252C_Veenpluis_(Eriophorum_angustifolium)_03.JPG.webp)
With increased acidification in freshwater ecosystems, there will be a decrease in biodiversity, with the increased loss of acid-sensitive species.[10] A fall in pH to 6 would drastically affect both snail and crustacean's species within freshwater.[10] For example, within the Norwegian lakes, these species represent 45% of the trout's food source, resulting in a 10-30% reduction in trout due to freshwater acidification.[10] In addition, zooplankton's species diversity is affected by freshwater acidification.[11]
In most acidic freshwater reservoirs, there will be an increase in the development of mosses and algae.[10] In particular, it is common to see an increase in the abundance of the moss Sphagnum.[10] Sphagnum has a high capacity to exchange H+ for basic cations within freshwater.[10] The thick layer of Sphagnum is restricting the exchange between surface water and sediment, which further contributes to reduction in nutrient cycling in the ecosystem.[10]
References
- Psenner, Roland (March 1994). "Environmental impacts on freshwaters: acidification as a global problem". Science of the Total Environment. 143 (1): 53–61. Bibcode:1994ScTEn.143...53P. doi:10.1016/0048-9697(94)90532-0. ISSN 0048-9697.
- Irwin, J.G.; Williams, M.L. (1988). "Acid rain: Chemistry and transport". Environmental Pollution. 50 (1–2): 29–59. doi:10.1016/0269-7491(88)90184-4. ISSN 0269-7491. PMID 15092652.
- Jean-Pierre Gattuso; Lina Hansson, eds. (2011). Ocean acidification. Oxford University Press. ISBN 9780199591084. OCLC 975179973.
- "Measurements and observations : OCB-OA". www.whoi.edu. Retrieved 2019-03-24.
- Cardoso, A.C.; Free, G.; Nõges, P.; Kaste, Ø.; Poikane, S.; Solheim, A. Lyche (2009). "Lake Management, Criteria". Encyclopedia of Inland Waters. Elsevier. pp. 310–331. doi:10.1016/b978-012370626-3.00244-1. ISBN 9780123706263.
- Henriksen, Arne; Kämäri, Juha; Posch, Maximilian; Wilander, Anders (1992). "Critical Loads of Acidity: Nordic Surface Waters". Ambio. 21 (5): 356–363. ISSN 0044-7447. JSTOR 4313961.
- Clair, Thomas A.; Dennis, Ian F.; Scruton, David A.; Gilliss, Mallory (December 2007). "Freshwater acidification research in Atlantic Canada: a review of results and predictions for the future". Environmental Reviews. 15 (NA): 153–167. doi:10.1139/a07-004. ISSN 1181-8700.
- Weiss, Linda C.; Pötter, Leonie; Steiger, Annika; Kruppert, Sebastian; Frost, Uwe; Tollrian, Ralph (January 2018). "Rising pCO2 in Freshwater Ecosystems Has the Potential to Negatively Affect Predator-Induced Defenses in Daphnia". Current Biology. 28 (2): 327–332.e3. doi:10.1016/j.cub.2017.12.022. ISSN 0960-9822. PMID 29337079.
- Hasler, Caleb T.; Butman, David; Jeffrey, Jennifer D.; Suski, Cory D. (January 2016). Sterner, Robert (ed.). "Freshwater biota and rising pCO 2 ?". Ecology Letters. 19 (1): 98–108. doi:10.1111/ele.12549. PMID 26610406.
- "The Effect of Coal Utilization Emissions on Natural and Man-managed Terrestrial and Freshwater Ecosystems". Environmental Impacts of Coal Mining & Utilization. Elsevier. 1987. pp. 282–318. doi:10.1016/b978-0-08-031427-3.50020-7. ISBN 9780080314273.
- Muniz, Ivar P. (1990). "Freshwater acidification: its effects on species and communities of freshwater microbes, plants and animals". Proceedings of the Royal Society of Edinburgh. Section B. Biological Sciences. 97: 227–254. doi:10.1017/s0269727000005364. ISSN 0269-7270.