Fujimoto–Belleau reaction
The Fujimoto–Belleau reaction is a chemical reaction that forms cyclic α-substituted α,β-unsaturated ketones from enol lactones. The reaction is named after the two chemists George I. Fujimoto and Bernard Belleau.
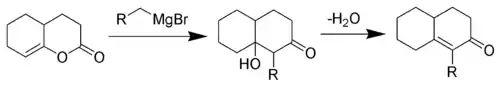
The Fujimoto-Belleau reaction
The reaction is a Grignard reaction, followed by a H-shift, an enol-keto tautomerisation and an Aldol addition reaction. The last step is an elimination (Aldol condensation) reaction with an E1CB mechanism.
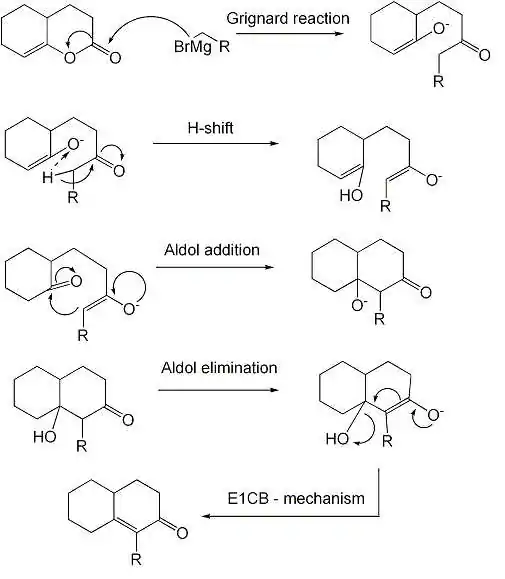
mechanism of the reaction
References
- George I. Fujimoto (1951). "Labeling of Steroids in the 4-Position". J. Am. Chem. Soc. 73 (4): 1856. doi:10.1021/ja01148a518.
- Bernard Belleau (1951). "The Reaction of Methylmagnesium Iodide with β-(1-Hydroxy-3,4-dihydro-2-naphthyl)-butyric Acid Lactone". J. Am. Chem. Soc. 73 (11): 5441–5443. doi:10.1021/ja01155a504.
- Weill-Raynal, J. Synthesis 1969, 49.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.