GPCR oligomer
A GPCR oligomer is a protein complex that consists of a small number (ὀλίγοι oligoi "a few", μέρος méros "part, piece, component") of G protein-coupled receptors (GPCRs). It is held together by covalent bonds or by intermolecular forces. The subunits within this complex are called protomers, while unconnected receptors are called monomers. Receptor homomers consist of identical protomers, while heteromers consist of different protomers.
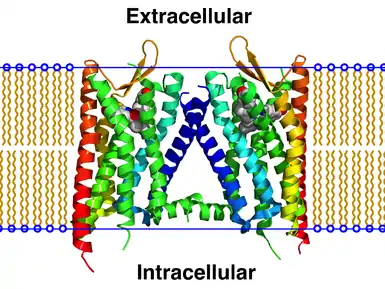
Receptor homodimers – which consist of two identical GPCRs – are the simplest homomeric GPCR oligomers. Receptor heterodimers – which consist of two different GPCRs – are the simplest heteromeric GPCR oligomers.
The existence of receptor oligomers is a general phenomenon, whose discovery has superseded the prevailing paradigmatic concept of the function of receptors as plain monomers, and has far-reaching implications for the understanding of neurobiological diseases as well as for the development of drugs.[2][3]
Discovery
For a long time it was assumed that receptors transmitted their effects exclusively from their basic functional forms – as monomers. The first clue to the existence of GPCR oligomers goes back to 1975 when Robert Lefkowitz observed that β-adrenoceptors display negative binding cooperativity.[4] At the beginning of the 1980s, it was hypothesized, receptors could form larger complexes, the so-called mosaic form,[5] where two receptors may interact directly with each other.[6] Mass determination of β-adrenoceptors (1982)[7] and muscarinic receptors (1983),[8] supported the existence of homodimer or tetrameric complexes. In 1991, the phenomenon of receptor crosstalk was observed between adenosine A2A (A2A) and dopamine D2 receptor (DRD2) thus suggesting the formation of heteromers.[9] While initially thought to be a receptor heterodimer, a review from 2015 determined that the A2A-DRD2 heteromer is a heterotetramer composed of A2A and DRD2 homodimers (i.e., two adenosine A2A receptors and two dopamine D2 receptors).[10] Maggio and co-workers showed in 1993 the ability of the muscarinic M3 receptor and α2C-adrenoceptor to heterodimerize.[11] The first direct evidence that GPCRs functioned as oligomers in vivo came from Overton and Blumer in 2000 by fluorescence resonance energy transfer (FRET) analysis of the α-factor receptor in the yeast Saccharomyces cerevisiae.[12] In 2005, further evidence was provided that receptor oligomizeration plays a functional role in a living organism with regulatory implication.[13] The crystal structure of the CXCR4 dimer was published in 2010.[14]
Consequences of oligomerization
GPCR oligomers consist of receptor dimers, trimers, tetramers, and complexes of higher order. These oligomers are entities with properties that can differ from those of the monomers in several ways.[15] The functional character of a receptor is dependent on its tertiary or quaternary structure. Within the complex protomers act as allosteric modulators of another. This has consequences for:
- the supply of the cell surface with receptors
- the ligand binding at corresponding binding sites
- the G-protein coupling
- the GPCR-mediated signal transduction
- modifying the desensitization profile
- the tendency for endocytosis and internalization
- the post-endocytotic fate of the receptors
See also
References
- PDB: 4DJH; Wu H, Wacker D, Mileni M, Katritch V, Han GW, Vardy E, Liu W, Thompson AA, Huang XP, Carroll FI, Mascarella SW, Westkaemper RB, Mosier PD, Roth BL, Cherezov V, Stevens RC (March 2012). "Structure of the human κ-opioid receptor in complex with JDTic". Nature. 485 (7398): 327–32. doi:10.1038/nature10939. PMC 3356457. PMID 22437504.
- Albizu L, Moreno JL, González-Maeso J, Sealfon SC (November 2010). "Heteromerization of G protein-coupled receptors: relevance to neurological disorders and neurotherapeutics". CNS Neurol Disord Drug Targets. 9 (5): 636–50. doi:10.2174/187152710793361586. PMC 3066024. PMID 20632964.
- Rozenfeld R, Devi LA (March 2010). "Receptor heteromerization and drug discovery". Trends Pharmacol. Sci. 31 (3): 124–30. doi:10.1016/j.tips.2009.11.008. PMC 2834828. PMID 20060175.
- Limbird LE, Meyts PD, Lefkowitz RJ (June 1975). "Beta-adrenergic receptors: evidence for negative cooperativity". Biochem. Biophys. Res. Commun. 64 (4): 1160–8. doi:10.1016/0006-291x(75)90815-3. PMID 1137592.
- Fuxe K, Borroto-Escuela DO, Marcellino D, Romero-Fernandez W, Frankowska M, Guidolin D, Filip M, Ferraro L, Woods AS, Tarakanov A, Ciruela F, Agnati LF, Tanganelli S (2012). "GPCR heteromers and their allosteric receptor-receptor interactions". Curr. Med. Chem. 19 (3): 356–63. doi:10.2174/092986712803414259. PMID 22335512.
- Birdsall NJM (1982). "Can different receptors interact directly with each other?". Trends in Neurosciences. 5: 137–138. doi:10.1016/0166-2236(82)90081-9.
- Fraser CM, Venter JC (November 1982). "The size of the mammalian lung beta 2-adrenergic receptor as determined by target size analysis and immunoaffinity chromatography". Biochem. Biophys. Res. Commun. 109 (1): 21–9. doi:10.1016/0006-291x(82)91560-1. PMID 6297476.
- Avissar S, Amitai G, Sokolovsky M (January 1983). "Oligomeric structure of muscarinic receptors is shown by photoaffinity labeling: subunit assembly may explain high- and low-affinity agonist states". Proc. Natl. Acad. Sci. U.S.A. 80 (1): 156–9. doi:10.1073/pnas.80.1.156. PMC 393329. PMID 6571990.
- Ferre S, von Euler G, Johansson B, Fredholm BB, Fuxe K (August 1991). "Stimulation of high-affinity adenosine A2 receptors decreases the affinity of dopamine D2 receptors in rat striatal membranes" (PDF). Proc. Natl. Acad. Sci. U.S.A. 88 (16): 7238–41. doi:10.1073/pnas.88.16.7238. PMC 52269. PMID 1678519.
- Ferré S, Bonaventura J, Tomasi D, Navarro G, Moreno E, Cortés A, Lluís C, Casadó V, Volkow ND (June 2015). "Allosteric mechanisms within the adenosine A2A-dopamine D2 receptor heterotetramer". Neuropharmacology. 104: 154–60. doi:10.1016/j.neuropharm.2015.05.028. PMC 5754196. PMID 26051403.
- Maggio R, Vogel Z, Wess J (April 1993). "Coexpression studies with mutant muscarinic/adrenergic receptors provide evidence for intermolecular "cross-talk" between G-protein-linked receptors". Proc. Natl. Acad. Sci. U.S.A. 90 (7): 3103–7. doi:10.1073/pnas.90.7.3103. PMC 46245. PMID 8385357.
- Overton MC, Blumer KJ (2000). "G-protein-coupled receptors function as oligomers in vivo". Curr. Biol. 10 (6): 341–4. doi:10.1016/S0960-9822(00)00386-9. PMID 10744981.
- Waldhoer M, Fong J, Jones RM, Lunzer MM, Sharma SK, Kostenis E, Portoghese PS, Whistler JL (June 2005). "A heterodimer-selective agonist shows in vivo relevance of G protein-coupled receptor dimers". Proc. Natl. Acad. Sci. U.S.A. 102 (25): 9050–5. doi:10.1073/pnas.0501112102. PMC 1157030. PMID 15932946.
- Wu B, Chien EY, Mol CD, Fenalti G, Liu W, Katritch V, Abagyan R, Brooun A, Wells P, Bi FC, Hamel DJ, Kuhn P, Handel TM, Cherezov V, Stevens RC (November 2010). "Structures of the CXCR4 chemokine GPCR with small-molecule and cyclic peptide antagonists". Science. 330 (6007): 1066–71. doi:10.1126/science.1194396. PMC 3074590. PMID 20929726.
- Wnorowski, A; Jozwiak, K (2014). "Homo- and hetero-oligomerization of β2-adrenergic receptor in receptor trafficking, signaling pathways and receptor pharmacology". Cellular Signalling. 26 (10): 2259–65. doi:10.1016/j.cellsig.2014.06.016. PMID 25049076.
- Guo H, An S, Ward R, Yang Y, Liu Y, Guo XX, Hao Q, Xu TR (2017). "Methods used to study the oligomeric structure of G-protein-coupled receptors". Biosci. Rep. 37 (2). doi:10.1042/BSR20160547. PMC 5398257. PMID 28062602.
- Cottet M, Faklaris O, Maurel D, Scholler P, Doumazane E, Trinquet E, Pin JP, Durroux T (2012). "BRET and Time-resolved FRET strategy to study GPCR oligomerization: from cell lines toward native tissues". Front Endocrinol (Lausanne). 3: 92. doi:10.3389/fendo.2012.00092. PMC 3401989. PMID 22837753.
Further reading
- Smith NJ, Milligan G (December 2010). "Allostery at G protein-coupled receptor homo- and heteromers: uncharted pharmacological landscapes". Pharmacol. Rev. 62 (4): 701–25. doi:10.1124/pr.110.002667. PMC 2993260. PMID 21079041.
- González-Maeso J (2011). "GPCR oligomers in pharmacology and signaling". Mol Brain. 4 (1): 20. doi:10.1186/1756-6606-4-20. PMC 3128055. PMID 21619615.
- Saenz del Burgo L, Milligan G (2011). "Ligand Regulation of GPCR Quaternary Structure". In Giraldo J, Pin JP (eds.). G protein-coupled receptors: from structure to function. Cambridge: Royal Society of Chemistry. pp. 111–152. ISBN 978-1-84973-183-6.
- Gilchrist A (2010). GPCR molecular pharmacology and drug targeting: shifting paradigms and new directions. Hoboken, N.J.: John Wiley & Sons. ISBN 978-0-470-30778-6.
- Borroto-Escuela DO, Brito I, Romero-Fernandez W, et al. (2014). "The G protein-coupled receptor heterodimer network (GPCR-HetNet) and its hub components". Int J Mol Sci. 15 (5): 8570–90. doi:10.3390/ijms15058570. PMC 4057749. PMID 24830558.
External links
- "The G Protein Coupled Receptor - Oligomerization Knowledge Base Project". Weill Medical College of Cornell University. Archived from the original on 2012-04-28.
- "GRIP Server". Archived from the original on 2012-06-07. – Nemoto W, Fukui K, Toh H (December 2009). "GRIP: a server for predicting interfaces for GPCR oligomerization". J. Recept. Signal Transduct. Res. 29 (6): 312–7. doi:10.3109/10799890903295143. PMID 19888901.
- "GRIP Database". Archived from the original on 2012-06-10. – Nemoto W, Fukui K, Toh H (June 2011). "GRIPDB - G protein coupled Receptor Interaction Partners DataBase". J. Recept. Signal Transduct. Res. 31 (3): 199–205. doi:10.3109/10799893.2011.563312. PMID 21410407.