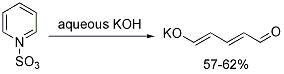Glutaconaldehyde
Glutaconaldehyde is an organic compound with the formula C5H6O2. It is an unsaturated dialdehyde as is related to glutaraldehyde and glutaconic acid, but exists in its enol form due to the conjugation with the double bond.
| Names | |
|---|---|
| IUPAC name
5-Hydroxy-2,4-pentadienal | |
| Other names
Glutacondialdehyde; Glutaconic aldehyde; Glutaconic dialdehyde; Pentenedial | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| C5H6O2 | |
| Molar mass | 98.101 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Both the sodium and potassium salts of glutaconaldehyde are known. They are efficiently synthesized from pyridinium sulfonate.[1][2]
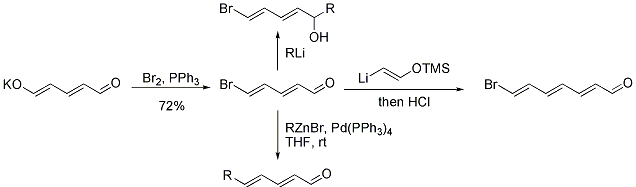
The reaction of the glutaconaldehyde anion with acid chlorides gives the corresponding enol esters.[1] Glutaconaldehyde can also be converted to the corresponding vinyl bromide as shown below.[3] This product can be reacted with nucleophiles, cross-coupled using palladium, or homologated by two carbons.[3][4]
References
- Baumgarten, P. (1924). "Über den Abbau des Pyridins zu Glutaconsäuredialdehyd und dessen Rückverwandlung in Pyridin (I.)". Ber. Dtsch. Chem. Ges. 57: 1622. doi:10.1002/cber.19240570876.
- Becher, J. (1979). "Glutaconaldehyde Sodium Salt from Hydrolysis of Pyridinium-1-Sulfonate". Organic Syntheses. 59: 79–84.
- Soullez, D.; Ple, G.; Duhamel, L. Duhamel P. (1995). "5-Bromopentadienal: A Versatile Intermediate for the Synthesis of Functionalized Polyenic Compounds". J. Chem. Soc., Chem. Commun.: 563–564.
- Vicart, N.; Castet-Caillabet, D.; Ramondenc, Y.; Ple, G.; Duhamel, L. (1998). "Application of (2E,4E)-5-Bromo-2,4-Pentadienal in Palladium Catalysed Cross-Coupling: Easy Access to (2E,4E)-2,4-Dienals". Synlett: 411–412.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.