HIV/AIDS research
HIV/AIDS research includes all medical research that attempts to prevent, treat, or cure HIV/AIDS, as well as fundamental research about the nature of HIV as an infectious agent and AIDS as the disease caused by HIV.
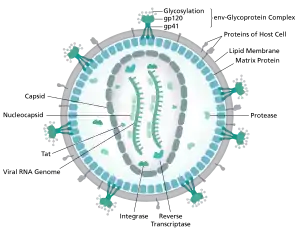
Transmission
A body of scientific evidence has shown that men who are circumcised are less likely to contract HIV than men who are uncircumcised.[1] Research published in 2014, concludes that the sex hormones estrogen and progesterone selectively impact HIV transmission.[2]
Pre- and post-exposure prophylaxis
"Pre-exposure prophylaxis" refers to the practice of taking some drugs before being exposed to HIV infection, and having a decreased chance of contracting HIV as a result of taking that drug. Post-exposure prophylaxis refers to taking some drugs quickly after being exposed to HIV, while the virus is in a person's body but before the virus has established itself. In both cases, the drugs would be the same as those used to treat persons with HIV, and the intent of taking the drugs would be to eradicate the virus before the person becomes irreversibly infected.
Post-exposure prophylaxis is recommended in anticipated cases of HIV exposure, such as if a nurse somehow has blood-to-blood contact with a patient in the course of work, or if someone without HIV requests the drugs immediately after having unprotected sex with a person who might have HIV. Pre-exposure prophylaxis is sometimes an option for HIV-negative persons who feel that they are at increased risk of HIV infection, such as an HIV-negative person in a serodiscordant relationship with an HIV-positive partner.
Current research in these agents include drug development, efficacy testing, and practice recommendations for using drugs for HIV prevention.
Within-host dynamics
The within-host dynamics of HIV infection include the spread of the virus in vivo, the establishment of latency, the effects of immune response on the virus, etc.[3][4] Early studies used simple models and only considered the cell-free spreading of HIV, in which virus particles bud from an infected T cell, enter the blood/extracellular fluid, and then infect another T cell.[4] A 2015 study[3] proposes a more realistic model of HIV dynamics that also incorporates the viral cell-to-cell spreading mechanism, where the virus is directly transited from one cell to another, as well as the T cell activation, the cellular immune response, and the immune exhaustion as the infection progresses.[3]
Virus characteristics
HIV binds to immune cell surface receptors, including CD 4 and CXCR4 or CD4 and CCR5. The binding causes conformation changes and results in the membrane fusion between HIV and cell membrane. Active infection occurs in most cells, while latent infection occurs in much fewer cells 1, 2 and at very early stages of HIV infection. 9, 35 In active infection, HIV pro virus is active and HIV virus particles are actively replicated; and the infected cells continuously release viral progeny; while in latent infection, HIV pro virus is transcriptionally silenced and no viral progeny is produced.[5]
Management of HIV/AIDS
Research to improve current treatments includes decreasing side effects of current drugs, further simplifying drug regimens to improve adherence, and determining better sequences of regimens to manage drug resistance. There are variations in the health community in recommendations on what treatment doctors should recommend for people with HIV. One question, for example, is determining when a doctor should recommend that a patient take antiretroviral drugs and what drugs a doctor may recommend. This field also includes the development of antiretroviral drugs.
Age acceleration effects due to HIV-1 infection
Infection with the Human Immunodeficiency Virus-1 (HIV) is associated with clinical symptoms of accelerated aging, as evidenced by increased incidence and diversity of age-related illnesses at relatively young ages. A significant age acceleration effect could be detected in brain (7.4 years) and blood (5.2 years) tissue due to HIV-1 infection[6] with the help of a biomarker of aging, which is known as epigenetic clock.
Long-term nonprogressor
A long-term nonprogressor is a person who is infected with HIV, but whose body, for whatever reason, naturally controls the virus so that the infection does not progress to the AIDS stage. Such persons are of great interest to researchers, who feel that a study of their physiologies could provide a deeper understanding of the virus and disease.
HIV vaccine
An HIV vaccine is a vaccine that would be given to a person who does not have HIV, in order to confer protection against subsequent exposures to HIV, thus reducing the likelihood that the person would become infected by HIV. Currently, no effective HIV vaccine exists. Various HIV vaccines have been tested in clinical trials almost since the discovery of HIV.
Only a vaccine is thought to be able to halt the pandemic. This is because a vaccine would cost less, thus being affordable for developing countries, and would not require daily treatment.[7] However, after over 20 years of research, HIV-1 remains a difficult target for a vaccine.[7][8]
In 2003 a clinical trial in Thailand tested an HIV vaccine called RV 144. In 2009, the researchers reported that this vaccine showed some efficacy in protecting recipients from HIV infection (31% efficacy). Results of this trial give the first supporting evidence of any vaccine being effective in lowering the risk of contracting HIV. Other vaccine trials continue worldwide including a mosaic vaccine using an adenovirus 26 vector[9] as well as a newer formulation of RV144 called HVTN 702.[10]
One recent trial was conducted by scientists at The Scripps Research Institute (TSRI) who found a way to attach HIV-fighting antibodies to immune cells, creating a HIV-resistant cell population.
Microbicides for sexually transmitted diseases
A microbicide for sexually transmitted diseases is a gel which would be applied to the skin - perhaps a rectal microbicide for persons who engage in anal sex or a vaginal microbicide for persons who engage in vaginal sex - and if infected body fluid such as blood or semen were to touch the gel, then HIV in that fluid would be destroyed and the people having sex would be less likely to spread infection between themselves.
On March 7, 2013, the Washington University in St. Louis website published a report by Julia Evangelou Strait, in which it was reported that ongoing nanoparticle research showed that nanoparticles loaded with various compounds could be used to target infectious agents whilst leaving healthy cells unaffected. In the study detailed by this report, it was found that nanoparticles loaded with Mellitin, a compound found in Bee venom, could deliver the agent to the HIV, causing the breakdown of the outer protein envelope of the virus. This, they say, could lead to the production of a vaginal gel which could help prevent infection by disabling the virus.[11] Dr Joshua Hood goes on to explain that beyond preventive measures in the form of a topical gel, he sees "potential for using nanoparticles with melittin as therapy for existing HIV infections, especially those that are drug-resistant. The nanoparticles could be injected intravenously and, in theory, would be able to clear HIV from the blood stream."[11]
Stem cell transplantation
In 2007, Timothy Ray Brown,[12] a 40-year-old HIV-positive man, also known as "the Berlin Patient", was given a stem cell transplant as part of his treatment for acute myeloid leukemia (AML).[13] A second transplant was made a year later after a relapse. The donor was chosen not only for genetic compatibility but also for being homozygous for a CCR5-Δ32 mutation that confers resistance to HIV infection.[14][15] After 20 months without antiretroviral drug treatment, it was reported that HIV levels in Brown's blood, bone marrow, and bowel were below the limit of detection.[15] The virus remained undetectable over three years after the first transplant.[13] Although the researchers and some commentators have characterized this result as a cure, others suggest that the virus may remain hidden in tissues[16] such as the brain (which acts as a viral reservoir).[17] Stem cell treatment remains investigational because of its anecdotal nature, the disease and mortality risk associated with stem cell transplants, and the difficulty of finding suitable donors.[16][18]
Stem cell based gene therapy
In the past 7 years, scientists have been using different approaches of stem cell based gene therapy in an attempt to develop a cure as well as to propose an alternative to the conventional antiretroviral therapy (ART).[19] Specifically, advances had been made with a cure to HIV.
A cellular receptor, generally CCR5 or CXCR4 is required in order for HIV entry into CD4 cells. Cells of individuals homozygous for the CCR5 gene variant Δ32 (CCR5Δ32/Δ32) lack the CCR5 cell-surface expression, meaning that they are naturally resistant to infection with CCR5 tropic HIV strains (R5 HIV).[20] One study done in 2011 achieves successful CD4+ T-cell reconstitution as a result of CCR5Δ32/Δ32 stem cell transplantation at the systemic level and in the gut mucosal immune system in a patient with HIV. Additionally, it provides evidence for the reduction in the size of the potential HIV reservoir over time. The patient in this study even remained HIV free without any evidence of having it for more than 3.5 years.[21]
Other theoretical cures to HIV-1 have been proposed. One supposed cure to HIV-1 involves the creation of a disease-resistant immune system through transplantation of autologous, gene-modified (HIV-1-resistant) hematopoietic stem cells and progenitor cells (GM-HSPC). Though this study does involve several early stage clinical trials that have demonstrated the safety and feasibility of this technique only for HIV-1, none have resulted in improvement of the disease state itself.[22] Therefore, this strategy is intended to go alongside already existing treatment techniques such as drugs and vaccines. However, future technology regarding this approach of single treatment cell therapy could potentially replace current therapy altogether as a functional or sterilizing cure to HIV-1.[22]
An additional study involves the use of genetically engineered CD34+ hematopoietic stem and progenitor cells. Experimental long-term in vivo HIV gene therapy have had huge issues due to both transduction ending in multiple copies of heterologous DNA in target cells as well as low efficacy of cell transduction at the time of transplantation. This study demonstrated the efficacy of a transplantation approach that ultimately allows for an enriched population of HSPCs expressing a single copy of a CCR5 miRNA.[23] Since positive selection of modified cells is likely to be insufficient below the threshold they found of at least 70% of the HIV target cells resulting in gene modification from efficient maintenance of CD34+ T cell and a low viral titer, the findings show evidence that clinical protocols of HIV gene therapy require a selective enrichment of genetically targeted cells.[23]
Immunomodulatory agents
Complementing efforts to control viral replication, immunotherapies that may assist in the recovery of the immune system have been explored in past and ongoing trials, including IL-2 and IL-7.[24]
The failure of vaccine candidates to protect against HIV infection and progression to AIDS has led to a renewed focus on the biological mechanisms responsible for HIV latency. A limited period of therapy combining anti-retrovirals with drugs targeting the latent reservoir may one day allow for total eradication of HIV infection.[25] Researchers have discovered an abzyme that can destroy the protein gp120 CD4 binding site. This protein is common to all HIV variants as it is the attachment point for B lymphocytes and subsequent compromising of the immune system.[26]
New developments
A turning point for HIV research occurred in 2007, following the bone marrow transplant of HIV sufferer Timothy Ray Brown. Brown underwent the procedure after he developed leukaemia and the donor of the bone marrow possessed a rare genetic mutation that caused Brown's cells to become resistant to HIV. Brown attained the title of the "Berlin Patient" in the HIV research field and is the first man to have been cured of the virus. As of April 2013, two primary approaches are being pursued in the search for a HIV cure: The first is gene therapy that aims to develop a HIV-resistant immune system for patients, and the second is being led by Danish scientists, who are conducting clinical trials to strip the HIV from human DNA and have it destroyed permanently by the immune system.[27]
Three more cases with similarities to the Brown case have occurred since the 2007 discovery; however, they differ because the transplanted marrow has not been confirmed as mutated. Two of the cases were publicized in a July 2013 CNN story that relayed the experience of two patients who had taken antiretroviral therapy for years before they developed lymphoma, a cancer of the lymph nodes. They then underwent lymphoma chemotherapy and bone marrow transplantation, while remaining on an antiretroviral regimen; while they retained traces of HIV four months afterwards, six to nine months after the transplant, the two patients had no detectable trace of HIV in their blood. However, the managing clinician Dr. Timothy Heinrich stated at the Malaysian International AIDS Society Conference where the findings were presented:
It's possible, again, that the virus could return in a week, it could return in a month -- in fact, some mathematical modeling predicts that virus could even return one to two years after we stop antiretroviral therapy, so we really don't know what the long-term or full effects of stem cell transplantation and viral persistence is.[28]
In March 2016, researchers at Temple University, Philadelphia, reported that they have used genome editing to delete HIV from T cells. According to the researchers, this approach could lead to a dramatic reduction of the viral load in patient cells.[29][30]
In April 2016, Innovative Bioresearch,[31] a privately held company owned by research scientist Jonathan Fior, reported the results of a pioneering pilot study that explored the infusion of SupT1 cells as a cell-based therapy for HIV in a humanized mouse model.[32][33] This novel cell-based therapy uses irradiated SupT1 cells as a decoy target for HIV to prevent CD4+ T cell depletion as well as to render the virus less cytopathic. The research showed that in animals treated with SupT1 cell infusion, significantly lower plasma viral load (~10-fold) and potentially preserved CD4+ T cell frequency were observed at Week 1, with one animal showing complete suppression of viral replication and preservation of CD4+ T cell count (no virus detected anymore at Weeks 3 and 4). As also mentioned in a previous paper written by the same author, Jonathan Fior, in vitro studies of HIV evolution showed that prolonged virus replication in the SupT1 cell line results in a less cytopathic virus with a reduced capacity for syncytium formation, a higher sensitivity to neutralization, improved replication in SupT1 cells and impaired infection of primary CD4+ T cell.[34] According to the research, this indicates that in vivo virus replication in the infused SupT1 cells should also have a vaccination effect.[33]
In March 2019, a patient with Hodgkin's lymphoma was also reported to possibly have been cured using similar treatment to Brown.[35]
See also
References
- CDC. "Male Circumcision".
- Diana Goode; Meropi Aravantinou; Sebastian Jarl; Rosaline Truong; Nina Derby; Natalia Guerra-Perez; Jessica Kenney; James Blanchard; Agegnehu Gettie; Melissa Robbiani; Elena Martinelli (May 15, 2014). "Sex Hormones Selectively Impact the Endocervical Mucosal Microenvironment: Implications for HIV Transmission". PLOS ONE. 9 (5): e97767. Bibcode:2014PLoSO...997767G. doi:10.1371/journal.pone.0097767. PMC 4022654. PMID 24830732.
- Zhang C; Zhou S; Groppelli E; Pellegrino P; Williams I; Borrow P; Chain BM; Jolly C (2015). "Hybrid Spreading Mechanisms and T Cell Activation Shape the Dynamics of HIV-1 Infection". PLOS Computational Biology. 11 (4): e1004179. arXiv:1503.08992. Bibcode:2015PLSCB..11E4179Z. doi:10.1371/journal.pcbi.1004179. PMC 4383537. PMID 25837979.
- Perelson AS; Ribeiro RM (2013). "Modeling the within-host dynamics of HIV infection". BMC Biology. 11 (1): 96. doi:10.1186/1741-7007-11-96. PMC 3765939. PMID 24020860.
- Ophinni, Youdiil; Inoue, Mari; Kotaki, Tomohiro; Kameoka, Masanori (17 May 2018). "CRISPR/Cas9 system targeting regulatory genes of HIV-1 inhibits viral replication in infected T-cell cultures". Scientific Reports. 8 (1): 7784. Bibcode:2018NatSR...8.7784O. doi:10.1038/s41598-018-26190-1. PMC 5958087. PMID 29773895.
- Horvath S; Levine AJ (2015). "HIV-1 infection accelerates age according to the epigenetic clock". J Infect Dis. 212 (10): 1563–73. doi:10.1093/infdis/jiv277. PMC 4621253. PMID 25969563.
- Ferrantelli F; Cafaro A; Ensoli B (December 2004). "Nonstructural HIV proteins as targets for prophylactic or therapeutic vaccine". Curr. Opin. Biotechnol. 15 (6): 543–56. doi:10.1016/j.copbio.2004.10.008. PMID 15560981.
- Karlsson Hedestam GB; Fouchier RA; Phogat S; Burton DR; Sodroski J; Wyatt RT (February 2008). "The challenges of eliciting neutralizing antibodies to HIV-1 and to influenza virus". Nat. Rev. Microbiol. 6 (2): 143–55. doi:10.1038/nrmicro1819. PMID 18197170.
- "HPX2008/ HVTN 705". AVAC. 2017-01-19. Archived from the original on 2019-01-22. Retrieved 2019-02-13.
- "HVTN 702". AVAC. 2015-04-17. Retrieved 2019-02-13.
- "Nanoparticles loaded with bee venom kill HIV". 7 March 2013.
- "German HIV patient cured after stem cell transplant". Belfast Telegraph. December 15, 2010. Retrieved December 15, 2010.
- Allers, K; Hütter, G; Hofmann, J; Loddenkemper, C; Rieger, K; Thiel, E; Schneider, T (2011-03-10). "Evidence for the cure of HIV infection by CCR5Δ32/Δ32 stem cell transplantation". Blood. 117 (10): 2791–9. doi:10.1182/blood-2010-09-309591. PMID 21148083.
- Mark Schoofs (November 7, 2008). "A Doctor, a Mutation and a Potential Cure for AIDS". The Wall Street Journal. Retrieved 2008-11-09.
- Hütter G; Nowak D; Mossner M; Ganepola S; Ganepola A; Allers K; Schneider T; Hofmann J; Kücherer C; Blau O; Blau IW; Hofmann WK; Thiel E (2009). "Long-Term Control of HIV by CCR5 Delta32/Delta32 Stem-Cell Transplantation". N Engl J Med. 360 (7): 692–698. doi:10.1056/NEJMoa0802905. PMID 19213682.
- Levy JA (2009). "Not an HIV Cure, but Encouraging New Directions". N Engl J Med. 360 (7): 724–725. doi:10.1056/NEJMe0810248. PMID 19213687.
- Nath, A; Clements, JE (2011-03-13). "Eradication of HIV from the brain: reasons for pause". AIDS. 25 (5): 577–80. doi:10.1097/QAD.0b013e3283437d2f. PMC 3681810. PMID 21160414.(subscription required)
- Lunzen, J.; Fehse, B.; Hauber, J. (2011). "Gene Therapy Strategies: Can We Eradicate HIV?". Current HIV/AIDS Reports. 8 (2): 78–84. doi:10.1007/s11904-011-0073-9. PMID 21331536.(subscription required)
- Younan, Patrick (2014). "Genetically Modified Hematopoietic Stem Cell Transplantation for HIV-1–infected Patients: Can We Achieve a Cure?". Molecular Therapy. 22 (2): 257–64. doi:10.1038/mt.2013.264. PMC 3916050. PMID 24220323.
- Dean, Michael (1996-09-27). "Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene". Science. 273 (5283): 1856–1862. Bibcode:1996Sci...273.1856D. doi:10.1126/science.273.5283.1856. PMID 8791590.
- Schneider, Thomas (2011-03-10). "Evidence for the cure of HIV infection by CCR532/32 stem cell transplantation". Blood. 117 (10): 2791–2799. doi:10.1182/blood-2010-09-309591. PMID 21148083.
- DiGiusto, David (2013-11-05). "Development of hematopoietic stem cell based gene therapy for HIV-1 infection: considerations for proof of concept studies and translation to standard medical practice". Viruses. 5 (11): 2898–919. doi:10.3390/v5112898. PMC 3856421. PMID 24284880.
- Myburgh, Renier (2015). "Lentivector Knockdown of CCR5 in Hematopoietic Stem and Progenitor Cells Confers Functional and Persistent HIV-1 Resistance in Humanized Mice". Journal of Virology. 89 (13): 6761–6772. doi:10.1128/JVI.00277-15. PMC 4468501. PMID 25903342.
- Tincati, C; d'Arminio Monforte, A; Marchetti, G (January 2009). "Immunological mechanisms of interleukin-2 (IL-2) treatment in HIV/AIDS disease". Current Molecular Pharmacology. 2 (1): 40–5. doi:10.2174/1874467210902010040. PMID 20021444.
- Bowman MC; Archin NM; Margolis DM (2009). "Pharmaceutical approaches to eradication of persistent HIV infection". Expert Reviews in Molecular Medicine. 11 (e6): e6. doi:10.1017/S1462399409000970. PMID 19208267.
- Planque S; Nishiyama Y; Taguchi H; Salas M; Hanson C; Paul S (June 2008). "Catalytic antibodies to HIV: Physiological role and potential clinical utility". Autoimmun Rev. 7 (6): 473–9. doi:10.1016/j.autrev.2008.04.002. PMC 2527403. PMID 18558365.
- Jake Wallis Simons (27 April 2013). "Scientists on brink of HIV cure". The Telegraph. Retrieved 2 May 2013.
- Saundra Young (3 July 2013). "Patients HIV-free for now after transplant". CNN. Retrieved 4 July 2013.
- Kaminski, Rafal; Chen, Yilan; Fischer, Tracy; Tedaldi, Ellen; Napoli, Alessandro; Zhang, Yonggang; Karn, Jonathan; Hu, Wenhui; Khalili, Kamel (4 March 2016). "Elimination of HIV-1 Genomes from Human T-lymphoid Cells by CRISPR/Cas9 Gene Editing". Scientific Reports. 6: 22555. Bibcode:2016NatSR...622555K. doi:10.1038/srep22555. PMC 4778041. PMID 26939770.
- Dvorsky, George (22 March 2016). "HIV Genes Successfully Edited Out of Immune Cells". Gizmodo. Retrieved 23 March 2016.
- "HIV And Cancer Research | Innovative Bioresearch". HIV And Cancer Research | Innovative Bioresearch (in Italian). Retrieved 2018-01-15.
- "Innovative Bioresearch Announces Publication of Pioneering Pilot Study Exploring SupT1 Cell Infusion as a Cell-Based Therapy for HIV in Humanized Mice". www.prnewswire.com. April 26, 2016.
- Fior, Jonathan (2016). "SupT1 Cell Infusion as a Possible Cell-Based Therapy for HIV: Results from a Pilot Study in Hu-PBMC BRGS Mice". Vaccines. 4 (2): 13. doi:10.3390/vaccines4020013. PMC 4931630. PMID 27128948.
- Fior, Jonathan (2013). "Is a Pacific Coexistence Between Virus and Host the Unexploited Path That May Lead to an HIV Functional Cure?". Viruses. 5 (2): 753–757. doi:10.3390/v5020753. PMC 3640524. PMID 23430684.
- Brueck, Hilary (6 March 2019). "Scientists may have cured a man of HIV by injecting him with 'superpower' cells. Here's what it means for the future of AIDS treatment". Business Insider. Retrieved 6 March 2019.