Hawkinsin
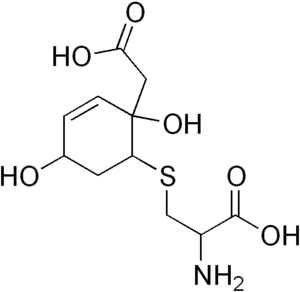
Hawkinsin (also known as 2-cystenyl-1,4-dihydroxycyclohexenylacetate[1]) is an amino acid, which is formed after detoxification of a reactive tyrosine metabolite (quinol acetate) by glutathione. Hawkinsin is ninhydrin positive (a common test to detect amino acids and proteins with a free -NH2 group).
 | |
| Names | |
|---|---|
| IUPAC name
2-Amino-3-[[2-(carboxymethyl)-2,5-dihydroxy-1-cyclohex-3-enyl]sulfanyl]propanoic acid | |
| Other names
(2-L-Cystein-S-yl-1,4-dihydroxycyclohex-5-en-1-yl)acetic acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
PubChem CID |
|
| |
| Properties | |
| C11H17NO6S | |
| Molar mass | 291.32 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
It is found in elevated concentrations in the urine in hawkinsinuria, which is probably related to the depletion of glutathione and resulting high excretion of 5-oxoproline.[2]
References
- Physician's guide to the diagnosis, treatment, and follow-up of inherited metabolic diseases. Blau, N. (Nenad), 1946-, Duran, Martinus,, Gibson, K. Michael,, Dionisi-Vici, Carlo. Berlin. 2014-07-08. ISBN 9783642403378. OCLC 874142358.CS1 maint: others (link)
- Physician's guide to the diagnosis, treatment, and follow-up of inherited metabolic diseases. Blau, N. (Nenad), 1946-, Duran, Martinus,, Gibson, K. Michael,, Dionisi-Vici, Carlo. Berlin. 2014-07-08. ISBN 9783642403378. OCLC 874142358.CS1 maint: others (link)
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.