Hexamethylene diisocyanate
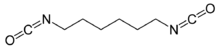
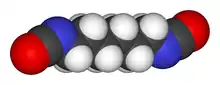
Hexamethylene diisocyanate (HDI) is the organic compound with the formula (CH2)6(NCO)2. It is classified as an diisocyanate. It is a colorless liquid.[2]
 | |
 | |
| Names | |
|---|---|
| IUPAC name
1,6-diisocyanatohexane | |
| Other names
HDI 1,6-hexane diisocyanate | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.011.350 |
PubChem CID |
|
| UNII |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C8H12N2O2 | |
| Molar mass | 168.2 g/mol |
| Appearance | Colourless liquid |
| Odor | sharp, pungent[1] |
| Density | 1.047 g/cm3, liquid |
| Melting point | −67 °C (−89 °F; 206 K) |
| Boiling point | 255 °C (491 °F; 528 K) |
| Vapor pressure | 0.05 mmHg (25 °C)[1] |
| Viscosity | 3 cP at 25 °C |
| Hazards | |
| Flash point | 130–140 °C (Cleveland open cup) |
| NIOSH (US health exposure limits): | |
PEL (Permissible) |
none[1] |
REL (Recommended) |
TWA 0.005 ppm (0.035 mg/m3) C 0.020 ppm (0.140 mg/m3) [10-minute][1] |
IDLH (Immediate danger) |
N.D.[1] |
| Related compounds | |
Related isocyanates |
Isophorone diisocyanate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Synthesis
Compared to other commercial diisocyanates, HDI is produced in relatively small quantities, accounting for (with isophorone diisocyanate) only 3.4% of the global diisocyanate market in the year 2000.[3] It is produced by phosgenation of hexamethylene diamine.
Applications
Aliphatic diisocyanates are used in specialty applications, such as enamel coatings which are resistant to abrasion and degradation by ultraviolet light. These properties are particularly desirable in, for instance, the exterior paint applied to aircraft and vessels. HDI is also sold oligomerized as the trimer or biuret. Although more viscous in these forms, it reduces the volatility and toxicity. At least 3 companies sell material in this form commercially.
References
- NIOSH Pocket Guide to Chemical Hazards. "#0320". National Institute for Occupational Safety and Health (NIOSH).
- Christian Six, Frank Richter (2005). "Isocyanates, Organic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a14_611.CS1 maint: uses authors parameter (link)
- Randall, David; Lee, Steve (2002). The Polyurethanes Book. New York: Wiley. ISBN 978-0-470-85041-1.
External links
- NIOSH Safety and Health Topic: Isocyanates, from the website of the National Institute for Occupational Safety and Health (NIOSH)
- NIOSH Pocket Guide to Chemical Hazards - Hexamethylene diisocyanate