Hexaphenylethane
Hexaphenylethane is a hypothetical organic compound consisting of an ethane core with six phenyl substituents. All attempts at its synthesis have been unsuccessful.[1] The trityl free radical, Ph3C · , was originally thought to dimerize to form hexaphenylethane. However, an inspection of the NMR spectrum of this dimer reveals that it is in fact a non-symmetrical species, Gomberg's dimer, rather than hexaphenylethane, due to the severe steric repulsions that hexaphenylethane would experience.
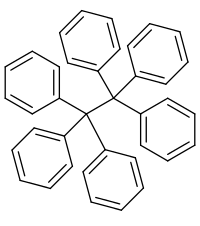 | |
| Names | |
|---|---|
| IUPAC name
1,1,1,2,2,2-Hexaphenylethane | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C38H30 | |
| Molar mass | 486.658 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
However, a substituted derivative of hexaphenylethane, hexakis(3,5-di-t-butylphenyl)ethane, has been prepared and features a very long central C–C bond at 167 pm (compared to the typical bond length of 154 pm). Attractive London dispersion forces between the t-butyl substituents are believed to be responsible for the stability of this very hindered molecule.[2]
See also
References
- Lewars, Errol G. (2008). "Chapter 8: Hexaphenylethane". Modeling Marvels. pp. 115–129. ISBN 9781402069734.
- Rösel, Sören; Balestrieri, Ciro; Schreiner, Peter R. (2017). "Sizing the role of London dispersion in the dissociation of all-meta tert-butyl hexaphenylethane". Chemical Science. 8 (1): 405–410. doi:10.1039/C6SC02727J. ISSN 2041-6520. PMC 5365070. PMID 28451185.
Literature
- Uchimura, Yasuto; Takeda, Takashi; Katoono, Ryo; Fujiwara, Kenshu; Suzuki, Takanori (2015). "Inside Back Cover: New Insights into the Hexaphenylethane Riddle: Formation of an α,o-Dimer". Angewandte Chemie International Edition. 54 (13): 4125. doi:10.1002/anie.201501649.