Hexasulfur
Hexasulfur (systematically named hexathiane, and cyclo-hexasulfur) is an inorganic chemical with the chemical formula S
6, consisting of a ring of six sulfur atoms. It is thus a simple cyclosulfane and an allotrope of sulfur. It is also the final member of the thiane heterocyclic series, where every carbon is substituted with a sulfur atom.
 | |
| Names | |
|---|---|
| Systematic IUPAC name
Hexathiane[1] | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| S6 | |
| Molar mass | 192.36 g·mol−1 |
| Appearance | Vivid, orange, opaque crystals |
| Related compounds | |
Related compounds |
Octasulfur |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Nomenclature
The name hexasulfur is the most commonly used and preferred IUPAC name and is constructed according to the compositional nomenclature. The systematic name hexathiane, a valid IUPAC name, is constructed according to the substitutive nomenclature. Another valid IUPAC systematic name cyclo-hexasulfur is constructed according to the additive nomenclature.
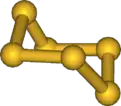
Structure
Hexasulfur adopts a chair configuration similar to that of cyclohexane, with bond angles of 102.2°. The sulfur atoms are equivalent.[2]
References
- "S6 - PubChem Public Chemical Database". The PubChem Project. USA: National Center for Biotechnology Information.
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 656. ISBN 978-0-08-037941-8.