Horsfiline
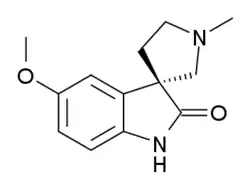
Horsfiline is an oxindole alkaloid found in the plant Horsfieldia superba,[1] which is used in traditional herbal medicine. It has analgesic effects and has been the subject of research both to produce it synthetically by convenient routes,[2][3][4][5] and to develop analogues and derivatives which may have improved analgesic effects.[6][7]
 | |
| Names | |
|---|---|
| IUPAC name
(3R)-5-methoxy-1'-methylspiro[1H-indole-3,3'-pyrrolidine]-2-one | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C13H16N2O2 | |
| Molar mass | 232.283 g·mol−1 |
| Melting point | 125 to 126 °C (257 to 259 °F; 398 to 399 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
It is a member of the spiroindolone class. Elacomine has a similar chemical structure.
References
- Jossang A, Jossang P, Hadi HA, Sevenet T, Bodo B (1991). "An oxindole alkaloid from Horsfieldia superba". Journal of Organic Chemistry. 56 (23): 6527–6530. doi:10.1021/jo00023a016.
- Lakshmaiah G, Kawabata T, Shang M, Fuji K (March 1999). "Total Synthesis of (-)-Horsfiline via Asymmetric Nitroolefination". The Journal of Organic Chemistry. 64 (5): 1699–1704. doi:10.1021/jo981577q. PMID 11674239.
- Cravotto G, Giovenzana GB, Pilati T, Sisti M, Palmisano G (December 2001). "Azomethine ylide cycloaddition/reductive heterocyclization approach to oxindole alkaloids: asymmetric synthesis of (-)-horsfiline". The Journal of Organic Chemistry. 66 (25): 8447–53. doi:10.1021/jo015854w. PMID 11735524.
- Murphy JA, Tripoli R, Khan TA, Mali UW (July 2005). "Novel phosphorus radical-based routes to horsfiline". Organic Letters. 7 (15): 3287–9. doi:10.1021/ol051095i. PMID 16018642.
- Trost BM, Brennan MK (May 2006). "Palladium asymmetric allylic alkylation of prochiral nucleophiles: horsfiline". Organic Letters. 8 (10): 2027–30. doi:10.1021/ol060298j. PMC 2565574. PMID 16671773.
- Tsai YC, Liou JP, Liao R, Cheng CY, Tao PL (July 1998). "C-alkylated spiro[benzofuran-3(2H),4'-1'-methyl-piperidine-7-ols] as potent opioids: a conformation-activity study". Bioorganic & Medicinal Chemistry Letters. 8 (14): 1813–8. doi:10.1016/S0960-894X(98)00318-7. PMID 9873439.
- Alf Claesson, Britt-Marie Swahn, Odd-Geir Berge. Spirooxindole derivatives that act as analgesics. US Patent 6774132
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.