Hydrazinium azide
Hydrazinium azide or hydrazine azide is a chemical compound with formula H
5N
5 or [N
2H+
5][N−
3]. It is a salt of the hydrazinium cation N
2H+
5 and the azide anion N−
3. It can be seen as a derivative of hydrazine N
2H
4 and hydrazoic acid HN
3. It is an unstable solid.
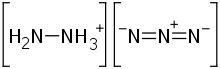 | |
| Names | |
|---|---|
| IUPAC name
Hydrazinium azide | |
| Identifiers | |
3D model (JSmol) |
|
PubChem CID |
|
| |
| |
| Properties | |
| H5N5 | |
| Molar mass | 75.075 g·mol−1 |
| Appearance | White solid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
The compound is of scientific interest because of its high nitrogen content and explosive properties.[1]
Structure
The solid undergoes structural phase transition to a different crystalline arrangement at a pressure of 13 GPa,[2]
Chemistry
Hydrazinium azide decomposes explosively into hydrazine, ammonia, and nitrogen gas:[3]
- 12 N
5H
5 → 3 N
2H
4 + 16 NH
3 + 19 N
2
Crystallization with an equimolar amount hydrazine yields the solid hydrazinium azide hydrazinate, [N
2H+
5][N−
3]·[N
2H
4], or N
7H
9, as monoclinic crystals. This compound is less hygroscopic and less volatile than pure hydrazinium azide. It decomposes explosively into nitrogen, hydrogen, and ammonia.[4]
At pressure of 40 GPa, hydrazinium azide decomposes yielding a linear nitrogen allotrope N
8 or N≡−−N=N−−≡N, that decomposes to ε-N2 below 25 GPa.[2]
Reaction of hydrazinium azide with sulfuric acid gives quantitative yields of pure hydrazinediium sulfate and hydrazoic acid:[5]
- [N
2H+
5][N−
3] + H2SO4 → [N
2H2+
6][SO2−
4] + HN3
See also
- Ammonium azide, [NH+
4][N−
3]
References
- Chiglien, G.; Etienne, J.; Jaulmes, S.; Laruelle, P. (15 September 1974). "Structure cristalline de l'azoture d'hydrazinium, N5H5". Acta Crystallographica Section B. 30 (9): 2229–2233. doi:10.1107/S0567740874006790.
- Duwal, Sakun; Ryu, Young-Jay; Kim, Minseob; Yoo, Choong-Shik; Bang, Sora; Kim, Kyungtae; Hur, Nam Hwi (7 April 2018). "Transformation of hydrazinium azide to molecular N8 at 40 GPa". The Journal of Chemical Physics. 148 (13): 134310. doi:10.1063/1.5021976. OSTI 1432864. PMID 29626901.
- G. B. Manelis (2003). Thermal decomposition and combustion of explosives and propellants. CRC Press. p. 235. ISBN 0-415-29984-5.
- Hammerl, Anton; Klapötke, Thomas M.; Piotrowski, Holger; Holl, Gerhard; Kaiser, Manfred (2001). "Synthesis and Characterization of Hydrazinium Azide Hydrazinate". Propellants, Explosives, Pyrotechnics. 26 (4): 161–164. doi:10.1002/1521-4087(200110)26:4<161::AID-PREP161>3.0.CO;2-O.
- Klapötke, T.; Peter S. White; Inis C. Tornieporth-Oetting (1996). "Reaction of hydrazinium azide with sulfuric acid: the X-ray structure of [N
2H
6][SO
4]". Polyhedron. 15 (15): 2579–2582. doi:10.1016/0277-5387(95)00527-7.