Hydrocortisone
Hydrocortisone is the name for the hormone cortisol when supplied as a medication.[3] Uses include conditions such as adrenocortical insufficiency, adrenogenital syndrome, high blood calcium, thyroiditis, rheumatoid arthritis, dermatitis, asthma, and COPD.[1] It is the treatment of choice for adrenocortical insufficiency.[4] It can be given by mouth, topically, or by injection.[1] Stopping treatment after long-term use should be done slowly.[1]
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | A-hydrocort, Cortef, Solu-cortef, others[1] |
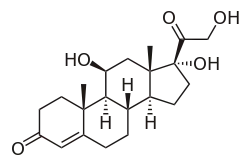
| Other names | Cortisol; 11β,17α,21-Trihydroxypregn-4-ene-3,20-dione |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682206 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth (tablets), intravenous, topical, rectal |
| Drug class | Corticosteroid; Glucocorticoid; Mineralocorticoid |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Elimination half-life | 1.5h[2] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C21H30O5 |
| Molar mass | 362.466 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Side effects may include mood changes, increased risk of infection, and swelling.[1] With long-term use common side effects include osteoporosis, upset stomach, physical weakness, easy bruising, and yeast infections.[1] While used, it is unclear if it is safe during pregnancy.[5] Hydrocortisone is a glucocorticoid and works as an anti-inflammatory and by immune suppression.[1]
Hydrocortisone was patented in 1936 and approved for medical use in 1941.[6][7] It is on the World Health Organization's List of Essential Medicines.[8] It is available as a generic medication.[1] In 2017, it was the 154th most commonly prescribed medication in the United States, with more than four million prescriptions.[9][10]
Medical uses
Hydrocortisone is the pharmaceutical term for cortisol used in oral administration, intravenous injection, or topical application. It is used as an immunosuppressive drug, given by injection in the treatment of severe allergic reactions such as anaphylaxis and angioedema, in place of prednisolone in patients needing steroid treatment but unable to take oral medication, and perioperatively in patients on long-term steroid treatment to prevent an adrenal crisis. It may also be injected into inflamed joints resulting from diseases such as gout.
It may be used topically for allergic rashes, eczema, psoriasis, itching and other inflammatory skin conditions. Topical hydrocortisone creams and ointments are available in most countries without prescription in strengths ranging from 0.05% to 2.5% (depending on local regulations) with stronger forms available by prescription only. Covering the skin after application increases the absorption and effect. Such enhancement is sometimes prescribed, but otherwise should be avoided to prevent overdose and systemic impact.
 Cortisol for injection
Cortisol for injection A tube of hydrocortisone cream, purchased over the counter
A tube of hydrocortisone cream, purchased over the counter
Pharmacology
Pharmacodynamics
Hydrocortisone is a corticosteroid, acting specifically as both a glucocorticoid and as a mineralocorticoid. That is, it is an agonist of the glucocorticoid and mineralocorticoid receptors.
Hydrocortisone has low potency relative to synthetic corticosteroids. Compared to hydrocortisone, prednisolone is about 4 times as potent and dexamethasone about 40 times as potent in terms of anti-inflammatory effect.[12] Prednisolone can also be used as cortisol replacement, and at replacement dose levels (rather than anti-inflammatory levels), prednisolone is about eight times more potent than cortisol.[13]
Pharmacokinetics
Most cortisol in the blood (all but about 4%) is bound to proteins, including corticosteroid binding globulin (CBG) and serum albumin. Free cortisol passes easily through cellular membranes, explaining its 100% bioavailability after oral administration.[14] Inside cells it interacts with corticosteroid receptors.[15]
Chemistry
Hydrocortisone, also known as 11β,17α,21-trihydroxypregn-4-ene-3,20-dione, is a naturally occurring pregnane steroid.[16][17] A variety of hydrocortisone esters exist and have been marketed for medical use.[16][17]
References
- "Hydrocortisone". Drugs.com. American Society of Health-System Pharmacists. February 9, 2015. Archived from the original on 20 September 2016. Retrieved 30 August 2016.
- Lennernäs H, Skrtic S, Johannsson G (2008). "Replacement therapy of oral hydrocortisone in adrenal insufficiency: The influence of gastrointestinal factors". Expert Opinion on Drug Metabolism & Toxicology. 4 (6): 749–58. doi:10.1517/17425255.4.6.749. PMID 18611115. S2CID 73248541.
- Becker KL (2001). Principles and Practice of Endocrinology and Metabolism. Lippincott Williams & Wilkins. p. 762. ISBN 9780781717502. Archived from the original on 2016-09-14.
- Hamilton R (2015). Tarascon Pocket Pharmacopoeia 2015 Deluxe Lab-Coat Edition. Jones & Bartlett Learning. p. 202. ISBN 9781284057560.
- "Hydrocortisone Pregnancy and Breastfeeding Warnings". Drugs.com. Archived from the original on 20 September 2016. Retrieved 1 September 2016.
- U.S. Patent 2,183,589
- Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 484. ISBN 9783527607495.
- World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- "The Top 300 of 2020". ClinCalc. Retrieved 11 April 2020.
- "Hydrocortisone - Drug Usage Statistics". ClinCalc. Retrieved 11 April 2020.
- WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group; Sterne JAC; Murthy, S.; Diaz, J. V.; Slutsky, A. S.; Villar, J.; Angus, D. C.; Annane, D.; Azevedo LCP; Berwanger, O.; Cavalcanti, A. B.; Dequin, P. F.; Du, B.; Emberson, J.; Fisher, D.; Giraudeau, B.; Gordon, A. C.; Granholm, A.; Green, C.; Haynes, R.; Heming, N.; Higgins JPT; Horby, P.; Jüni, P.; Landray, M. J.; Le Gouge, A.; Leclerc, M.; Lim, W. S.; Machado, F. R.; et al. (September 2, 2020). "Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients With COVID-19: A Meta-analysis". The Journal of the American Medical Association. 324 (13): 1330–1341. doi:10.1001/jama.2020.17023. PMC 7489434. PMID 32876694. S2CID 221467783.
- "Dexamethasone". drugs.com. Archived from the original on 21 June 2013. Retrieved 14 June 2013.
- Caldato MC, Fernandes VT, Kater CE (2004-10-01). "One-year clinical evaluation of single morning dose prednisolone therapy for 21-hydroxylase deficiency". Arquivos Brasileiros de Endocrinologia e Metabologia. 48 (5): 705–712. doi:10.1590/S0004-27302004000500017. ISSN 0004-2730. PMID 15761542.
- Charmandari E (2001). "Bioavailability of oral hydrocortisone in patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency". J Endocrinol. 169 (1): 65–70. doi:10.1677/joe.0.1690065. PMID 11250647.
- Boron WF, Boulpaep EL (2011). Medical Physiology (2nd ed.). Philadelphia: Saunders. ISBN 978-1-4377-1753-2.
- J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. p. 316. ISBN 978-1-4757-2085-3. Archived from the original on 8 September 2017.
- Index Nominum 2000: International Drug Directory. Taylor & Francis. 2000. pp. 524–. ISBN 978-3-88763-075-1.