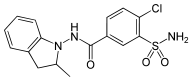
Indapamide
Indapamide is a thiazide-like diuretic[1] drug generally used in the treatment of hypertension, as well as decompensated heart failure. Combination preparations with perindopril (an ACE inhibitor antihypertensive) are also available. Thiazide-like diuretics (indapamide and chlorthalidone) appear to be more effective than the thiazide-type diuretics (hydrochlorothiazide) in reducing risks of major cardiovascular events and heart failure in persons with high blood pressure.[2] In terms of stroke risk, both thiazide-type and thiazide-like diuretics are effective in reducing it.[2][3][4] Both drug classes appear to have similar rates of adverse effects when compared to other classes of anti-hypertensives.[2]
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a684062 |
| Pregnancy category |
|
| Routes of administration | Oral tablet |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | 71–79% |
| Metabolism | Hepatic |
| Elimination half-life | 14–18 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.043.633 |
| Chemical and physical data | |
| Formula | C16H16ClN3O3S |
| Molar mass | 365.83 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
It was patented in 1968 and approved for medical use in 1977.[5]
Medical uses
Its indications include hypertension and edema due to congestive heart failure. Indapamide has been shown to reduce stroke rates in people over the age of 52.[6][7][8] When used in combination with perindopril, evidence from multiple studies show a greater reduction in stroke occurrence.[6][8] In the HYVET study, indapamide has been demonstrated to reduce all-cause mortality in octogenarians regardless of the addition of perindopril.[6]
Contraindications
Indapamide is contraindicated in known hypersensitivity to sulfonamides, severe kidney failure, hepatic encephalopathy or severe liver failure, and a low blood potassium level.
There is insufficient safety data to recommend indapamide use in pregnancy or breastfeeding.
Adverse effects
Commonly reported adverse events are low potassium levels, fatigue, orthostatic hypotension (an exaggerated decrease in blood pressure upon standing, often associated with syncope), and allergic manifestations.
Monitoring the serum levels of potassium and uric acid is recommended, especially in subjects with a predisposition to low levels of potassium in the blood and gout.
Interactions
Caution is advised in the combination of indapamide with lithium and drugs causing prolonged QT interval (on EKG) or wave-burst arrhythmia (ie: astemizole, bepridil, IV erythromycin, halofantrine, pentamidine, sultopride, terfenadine, and vincamine).
Overdose
Symptoms of over dosage would be those associated with a diuretic effect (ie electrolyte disturbances), low blood pressure, and muscular weakness. Treatment should be symptomatic, directed at correcting electrolyte abnormalities.
Dosage and administration
The adult dosage is 1.25 to 5 mg, orally and once daily, usually in the morning.
Indapamide is available generically as 1.25 mg and 2.5 mg non-scored tablets.[9] It is also available in SR (sustained release) form.
See also
References
- Indapamide at the US National Library of Medicine Medical Subject Headings (MeSH)
- Olde Engberink RH, Frenkel WJ, van den Bogaard B, Brewster LM, Vogt L, van den Born BJ (May 2015). "Effects of thiazide-type and thiazide-like diuretics on cardiovascular events and mortality: systematic review and meta-analysis". Hypertension. 65 (5): 1033–40. doi:10.1161/HYPERTENSIONAHA.114.05122. PMID 25733241.
- "Medical Research Council trial of treatment of hypertension in older adults: principal results. MRC Working Party". BMJ. 304 (6824): 405–12. February 1992. doi:10.1136/bmj.304.6824.405. PMC 1995577. PMID 1445513.
- Beckett NS, Peters R, Fletcher AE, Staessen JA, Liu L, Dumitrascu D, et al. (May 2008). "Treatment of hypertension in patients 80 years of age or older" (PDF). The New England Journal of Medicine. 358 (18): 1887–98. doi:10.1056/NEJMoa0801369. PMID 18378519.
- Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 457. ISBN 9783527607495.
- Beckett NS, Peters R, Fletcher AE, Staessen JA, Liu L, Dumitrascu D, et al. (May 2008). "Treatment of hypertension in patients 80 years of age or older" (PDF). The New England Journal of Medicine. 358 (18): 1887–98. doi:10.1056/NEJMoa0801369. PMID 18378519.
- Liu L, Wang Z, Gong L, Zhang Y, Thijs L, Staessen JA, Wang J (November 2009). "Blood pressure reduction for the secondary prevention of stroke: a Chinese trial and a systematic review of the literature". Hypertension Research. 32 (11): 1032–40. doi:10.1038/hr.2009.139. PMID 19798097.
- PROGRESS Collaborative Group (September 2001). "Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack". Lancet. 358 (9287): 1033–41. doi:10.1016/s0140-6736(01)06178-5. PMID 11589932.
- "Lexicomp Online Login". lexi.com.