J-aggregate
A J-aggregate is a type of dye with an absorption band that shifts to a longer wavelength (bathochromic shift) of increasing sharpness (higher absorption coefficient) when it aggregates under the influence of a solvent or additive or concentration as a result of supramolecular self-organisation.[1] The dye can be characterized further by a small Stokes shift with a narrow band. The J in J-aggregate refers to E.E. Jelley who discovered the phenomenon in 1936.[2][3] The dye is also called a Scheibe aggregate after G. Scheibe who also independently published on this topic in 1937.[4][5]
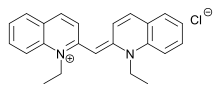
Scheibe and Jelley independently observed that in ethanol the dye PIC chloride has two broad absorption maxima at around 19,000 cm−1 and 20,500 cm−1 (526 and 488 nm respectively) and that in water a third sharp absorption maximum appears at 17,500 cm−1 (571 nm). The intensity of this band further increases on increasing concentration and on adding sodium chloride. In the oldest aggregation model for PIC chloride the individual molecules are stacked like a roll of coins forming a supramolecular polymer but the true nature of this aggregation phenomenon is still under investigation. Analysis is complicated because PIC chloride is not a planar molecule. The molecular axis can tilt in the stack creating a helix pattern. In other models the dye molecules orient themselves in a brickwork, ladder, or staircase fashion. In various experiments the J-band was found to split as a function of temperature, liquid crystal phases were found with concentrated solutions and CryoTEM revealed aggregate rods 350 nm long and 2.3 nm in diameter.
J-aggregate dyes are found with polymethine dyes in general, with cyanines, merocyanines, squaraine and perylene bisimides. Certain π-conjugated macrocycles, reported by Swager and co-workers at MIT, were also found to form J-aggregates and exhibited exceptionally high photoluminescence quantum yields.[6] Recently, a famous cyanine dye (TDBC) was reported to show enhanced photoluminescence quantum yield in the solution at room-temperature.[7]
See also
- H-aggregates, in which a hypsochromic shift is observed with low or no fluorescence.
References
- Würthner, F., Kaiser, T. E. and Saha-Möller, C. R. (2011), J-Aggregates: From Serendipitous Discovery to Supramolecular Engineering of Functional Dye Materials. Angewandte Chemie International Edition, 50: 3376–3410. doi:10.1002/anie.201002307
- Spectral Absorption and Fluorescence of Dyes in the Molecular State EDWIN E. JELLEY Nature 138, 1009-1010 (12 December 1936) doi:10.1038/1381009a0
- Nature 139, 631 (10 April 1937) | doi:10.1038/139631b0 Molecular, Nematic and Crystal States of I: I-Diethyl--Cyanine Chloride EDWIN E. JELLEY
- Naturwissenschaften Volume 25, Number 5, 75, doi:10.1007/BF01493278 Polymerisation und polymere Adsorption als Ursache neuartiger Absorptionsbanden von organischen Farbstoffen G. Scheibe, L. Kandler and H. Ecker
- Über die Veränderlichkeit der Absorptionsspektren in Lösungen und die Nebenvalenzen als ihre Ursache G. Scheibe Angewandte Chemie Volume 50, Issue 11, pages 212–219, 13. März 1937
- Chan, Julian M. W.; Tischler, Jonathan R.; Kooi, Steve E.; Bulovic, Vladimir; Swager, Timothy M. (2009). "Synthesis of J-Aggregating Dibenz[a,j]anthracene-Based Macrocycles". J. Am. Chem. Soc. 131 (15): 5659–5666. doi:10.1021/ja900382r. hdl:1721.1/74239. PMID 19326909.
- Anantharaman, Surendra B.; Kohlbrecher, Joachim; Rainò, Gabriele; Yakunin, Sergii; Stöferle, Thilo; Patel, Jay; Kovalenko, Maksym; Mahrt, Rainer F.; Nüesch, Frank A.; Heier, Jakob. "Enhanced Room-Temperature Photoluminescence Quantum Yield in Morphology Controlled J-Aggregates". Advanced Science. n/a (n/a): 1903080. doi:https://doi.org/10.1002/advs.201903080 Check
|doi=value (help).