Lawrence Que Jr.
Lawrence Que Jr. is a chemist who specialises in Inorganic chemistry and is currently a Regents Professor at the University of Minnesota.[1] He received the 2017 American Chemical Society (ACS) Award in Inorganic Chemistry for his contributions to the field.,[2] and the 2008 ACS Alfred Bader Award in Bioinorganic Chemistry.[3]
Biography
He obtained his B.S. degree in Chemistry from Ateneo de Manila University in Quezon City, Philippines in 1969. He then received his Ph.D. degree in Chemistry from the University of Minnesota in 1973 under the direction of Prof. Louis Pignolet. From there, Que went on to conduct postdoctoral studies with Professor Richard H. Holm at Massachusetts Institute of Technology followed by a research stint with Eckard Münck at Gray Freshwater Biological Institute from 1975 to 1977. His independent research career began at Cornell University where he held the position of Assistant Professor of Chemistry from 1977 until 1983. Que then moved back to the University of Minnesota where he currently is a Regents Professor.
Lawrence Que Jr. has published over 450 research manuscripts and 7 patents during his research career. He has presented almost 300 invited lectures and mentored almost 50 doctoral students. His inorganic chemistry research group at the University of Minnesota focuses on iron chemistry relevant to biocatalysis, in an attempt to better understand oxygen activation mechanisms of nonheme iron enzymes. His group is also working towards designing functional models for iron enzymes and capturing, observing, and categorizing highly active metal-based intermediates. He is also working on creating bio-inspired oxidation catalysts for green chemistry applications. For his contributions to the field of inorganic and bioinorganic chemistry, Que received the American Chemical Society's 2008 Alfred Bader Award in Bioinorganic Chemistry and 2017 Award in Inorganic Chemistry.
Bioinspired catalysis
Que has been studying the behaviors of high-valent iron-oxo species in relation to their ability of hydroxylation. Previous high-valent iron-oxo species have been noted and studied by observing [(Por•)Fe(IV)=O]+ in heme systems. However, it is yet to be established that a high-valent state could be accessed without a nonheme ligand environment. Que and his group studied various nonheme iron based complexes and through elaborate mechanistic work proved that Fe(V)=O species can indeed exist without the supporting heme ligand.
(TPA)(CH3CN)2.jpg.webp)
Nonheme iron oxygenases
_2%252C3-dioxygenase_with_heme_iron_center.jpg.webp)
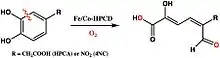
One of Que’s focuses is on the activation of dioxygen species in biological systems through non-heme iron active sites. More specifically, one of Que’s focuses is on homoprotocatechuate (HPCA) 2,3-dioxygenase, which mediates the electron transfer between catechol substrates and O2 to form a [M(II)(semiquinone)superoxo] intermediate.[4] Non-heme iron active sites, including those involving Mn(II) and Co(II), have proven to be equally potent as heme iron active sites with comparable or greater KMO2 and kcat.[5] By exploring nonheme iron oxygenases, Que hopes to optimize the first steps in the industrial production of methane gas, which would yield enormous energy savings for industry.
High-valent iron-oxo
Lawrence Que Jr has been recently working on creating synthetic oxoiron(IV) complexes with S=2 spin. The first crystallographic example of a high spin Fe(IV)=O was achieved in 2009,[6] and in 2011, Que proved that the ligand used in the previous study could also support a tricationic cyanoiron(IV) complex.[7]
References
- "Current Regents Professors". University of Minnesota Awards. Retrieved 2018-02-10.
- ACS Awards
- ACS Awards
- Fielding, Andrew J.; Kovaleva, Elena G.; Farquhar, Erik R.; Lipscomb, John D.; Que, Lawrence (February 2011). "A Hyperactive Cobalt-Substituted Extradiol-Cleaving Catechol Dioxygenase". Journal of Biological Inorganic Chemistry. 16 (2): 341–355. doi:10.1007/s00775-010-0732-0. ISSN 0949-8257. PMC 3192431. PMID 21153851.
- Fielding, Andrew J.; Lipscomb, John D.; Que, Lawrence (2012-01-18). "Characterization of an O2 Adduct of an Active Cobalt-Substituted Extradiol-Cleaving Catechol Dioxygenase". Journal of the American Chemical Society. 134 (2): 796–799. doi:10.1021/ja2095365. ISSN 0002-7863. PMC 3262093. PMID 22175783.
- England, J.; Martinho, M.; Farquhar, E. R.; Frisch, J. R.; Bominaar, E. L.; Münck, E.; Que, L. Jr. (2009). "A Synthetic High-Spin Oxoiron(IV) Complex: Generation, Spectroscopic Characterization, and Reactivity". Angew. Chem. Int. Ed. 48 (20): 3622–3626. doi:10.1002/anie.200900863. PMC 2719303. PMID 19373820.
- England, J.; Farquhar, E. R.; Guo, Y.; Cranswick, M. A.; Ray, K.; Münck, E.; Que, L. Jr. (2011). "Characterization of a Tricationic Trigonal Bipyramidal Iron(IV) Cyanide Complex, with a Very High Reduction Potential, and Its Iron(II) and Iron(III) Congeners". Inorg. Chem. 50 (7): 2885–2896. doi:10.1021/ic102094d. PMC 3065519. PMID 21381646.