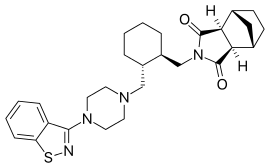
Lurasidone
Lurasidone, sold under the trade name Latuda among others, is an antipsychotic medication used to treat schizophrenia and bipolar disorder.[2] In bipolar it may be used together with a mood stabilizer such as lithium or valproate.[2] It is taken by mouth.[2]
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ljʊəˈræsɪdoʊn/ |
| Trade names | Latuda, others |
| Other names | SM-13496 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a611016 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| Drug class | Atypical antipsychotic[2] |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 9–19% (oral)[4] |
| Protein binding | ~99%[5] |
| Metabolism | Liver (CYP3A4-mediated)[4] |
| Elimination half-life | 18–40 hours[4][5] |
| Excretion | Faecal (67–80%), renal (9–19%)[4][5] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.225.187 |
| Chemical and physical data | |
| Formula | C28H36N4O2S |
| Molar mass | 492.68 g·mol−1 |
| 3D model (JSmol) | |
| Specific rotation | [α]20D −59° |
| Melting point | 176 to 178 °C (349 to 352 °F) |
| Solubility in water | 0.224 |
| |
| |
Common side effects include sleepiness, movement disorders, nausea, and diarrhea.[2] Serious side effects may include the potentially permanent movement disorder tardive dyskinesia, as well as neuroleptic malignant syndrome, an increased risk of suicide, angioedema, and high blood sugar levels.[2] In older people with psychosis as a result of dementia, it may increase the risk of dying.[2] Use during pregnancy is of unclear safety.[6][7] How it works is not clear but is believed to involve effects on dopamine and serotonin in the brain.[2]
Lurasidone was approved for medical use in the United States in 2010.[2] In 2019 generic versions were approved in the United States but will not be available until 2023.[8][9] In 2017, it was the 274th most commonly prescribed medication in the United States, with more than one million prescriptions.[10][11]
Medical uses
Lurasidone is used for the treatment of schizophrenia and bipolar disorder.[2]
In the United States, lurasidone is indicated for the treatment of:
- Schizophrenia in adults and adolescents (13 to 17 years)[12]
- Depressive episode associated with Bipolar I Disorder (bipolar depression) in adults and pediatric patients (10 to 17 years) as monotherapy[12]
- Depressive episode associated with Bipolar I Disorder (bipolar depression) in adults as adjunctive therapy with lithium or valproate[12]
In the European Union, lurasidone is indicated for the treatment of schizophrenia in adults aged 18 years and over.[13]
A 2014 review found lurasidone to be similar in effectiveness to other atypical antipsychotics.[14] A 2013 review of 15 antipsychotic medications in schizophrenia found lurasidone demonstrated mild effectiveness. It is considered to be as effective as iloperidone, and 13 to 15% less effective than ziprasidone, chlorpromazine, and asenapine.[15]
In July 2013, lurasidone received approval for bipolar I depression.[16][17][18][19] Few available atypical antipsychotics are known to possess antidepressant efficacy in bipolar disorder (with the notable exceptions being quetiapine,[20][21][22][23] olanzapine[24][25][26] and possibly asenapine[27]) as a monotherapy, even though the majority of atypical antipsychotics are known to possess significant antimanic activity,[28] which is yet to be clearly demonstrated for lurasidone.
Lurasidone is not approved by the Food and Drug Administration (FDA) for the treatment of behavior disorders in older adults with dementia.[29]
Contraindications
Lurasidone is contraindicated in individuals who are taking strong inhibitors of the liver enzyme CYP3A4 (ketoconazole, clarithromycin, ritonavir, levodropropizine, etc.) or inducers (carbamazepine, St. John's wort, phenytoin, rifampicin etc.).[30] The use of lurasidone in pregnant women has not been studied and is not recommended; in animal studies, no risks have been found.[31] Excretion in breast milk is also unknown; lurasidone is not recommended for breastfeeding women.[32] In the United States it is not indicated for use in children.[33]
Side effects
Side effects are generally similar to other antipsychotics. The drug has a relatively well tolerated side effect profile, with low propensity for QTc interval changes, weight gain and lipid-related adverse effects.[34] In a 2013 meta-analysis of the efficacy and tolerability of 15 antipsychotic drugs it was found to produce the second least (after haloperidol) weight gain, the least QT interval prolongation, the fourth most extrapyramidal side effects (after haloperidol, zotepine and chlorpromazine) and the sixth least sedation (after paliperidone, sertindole, amisulpride, iloperidone and aripiprazole).[15]
As with other atypical neuroleptics, lurasidone should be used with caution in the elderly because it puts them at an increased risk for a stroke or transient ischemic attack;[35][36] however, these risks are not likely to be greater than those associated with antipsychotics of other classes.[37] Similarly, lurasidone should not be used to treat dementia-related psychosis, as evidence has shown increased mortality with antipsychotic use.[38]
Weight gain is reported in up to 15 and 16 percent of users.[39][40]
Discontinuation
The British National Formulary recommends a gradual withdrawal when discontinuing antipsychotics to avoid acute withdrawal syndrome or rapid relapse.[41] Symptoms of withdrawal commonly include nausea, vomiting, and loss of appetite.[42] Other symptoms may include restlessness, increased sweating, and trouble sleeping.[42] Less commonly there may be a feeling of the world spinning, numbness, or muscle pains.[42] Symptoms generally resolve after a short period of time.[42]
There is tentative evidence that discontinuation of antipsychotics can result in psychosis.[43] It may also result in reoccurrence of the condition that is being treated.[44] Rarely tardive dyskinesia can occur when the medication is stopped.[42]
Interactions
Blood plasma concentrations may be increased when combined with CYP3A4 inhibitors, possibly leading to more side effects. This has been clinically verified for ketoconazole, which increases lurasidone exposure by a factor of 9, and is also expected for other 3A4 inhibitors such as grapefruit juice. Co-administration of CYP3A4 inducers like rifampicin or St. John's wort can reduce plasma levels of lurasidone and its active metabolite, and consequently decrease the effects of the drug. For rifampicin, the reduction was sixfold in a study.[5]
Pharmacology
Pharmacodynamics
| Site | Ki (nM) | Action | Species | Ref |
|---|---|---|---|---|
| SERT | >1,000 | ND | ND | [46] |
| NET | ND | ND | ND | ND |
| DAT | >1,000 | ND | ND | [46] |
| 5-HT1A | 6.75 | Partial agonist | Rat | [46] |
| 5-HT2A | 2.03 | Antagonist | Rat | [46] |
| 5-HT2B | ND | ND | ND | ND |
| 5-HT2C | 415 | ND | Pig | [46] |
| 5-HT3 | >1,000 | ND | ND | [46] |
| 5-HT4 | >1,000 | ND | ND | [46] |
| 5-HT7 | 0.495 | Antagonist | Human | [46] |
| α1 | 47.9 | ND | Rat | [46] |
| α2A | 40.7 | ND | Human | [46] |
| α2B | ND | ND | ND | ND |
| α2C | 10.8 | Antagonist | Human | [46] |
| β1 | >1,000 | ND | ND | [46] |
| β2 | >1,000 | ND | ND | [46] |
| D1 | 262 | ND | ND | [46] |
| D2 | 1.68 | Antagonist | Rat | [46] |
| D3 | 15.7 | Antagonist | ND | ND |
| D4.4 | 30 | ND | ND | ND |
| D5 | ND | ND | ND | ND |
| H1 | >1,000 | ND | Guinea pig | [46] |
| M1 | >1,000 | ND | Human | [46] |
| Values are Ki (nM). The smaller the value, the more strongly the drug binds to the site. | ||||
Lurasidone acts as an antagonist of the dopamine D2 and D3 receptors, the serotonin 5-HT2A and 5-HT7 receptors, and the α2C-adrenergic receptor, and as a partial agonist of the serotonin 5-HT1A receptor.[46][47] It has only low and likely clinically unimportant affinity for the serotonin 5-HT2C receptor, which may underlie its low propensity for appetite stimulation and weight gain.[46][48][49] The drug also has negligible affinity for the histamine H1 receptor and the muscarinic acetylcholine receptors, and hence has no antihistamine or anticholinergic effects.[47][50]
Pharmacokinetics
Lurasidone is taken by mouth and has an estimated absorption rate of 9 to 19%.[4] Studies have shown that when lurasidone is taken with food, absorption increases about twofold. Peak blood plasma concentrations are reached after one to three hours. About 99% of the circulating substance are bound to plasma proteins.[5]
Lurasidone is mainly metabolized in the liver via the enzyme CYP3A4, but has negligible affinity to other cytochrome P450 enzymes. It is transported by P-glycoprotein and ABCG2 and also inhibits these carrier proteins in vitro. It also inhibits the solute carrier protein SLC22A1, but no other relevant transporters.[5][35]
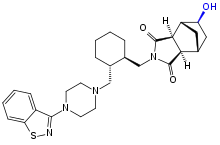
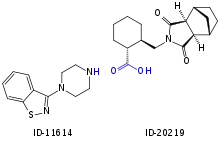
Main metabolism pathways are oxidative N-dealkylation between the piperazine and cyclohexane rings, hydroxylation of the norbornane ring, and S-oxidation.[5][53]:59 Other pathways are hydroxylation of the cyclohexane ring and reductive cleavage of the isothiazole ring followed by S-methylation.[52] The two relevant active metabolites are the norbornane hydroxylation products called ID-14283 and ID-14326, the former reaching pharmacologically relevant blood plasma concentrations. The two major inactive metabolites are the N-dealkylation products (the carboxylic acid ID-20219 and the piperazine ID-11614[52]), and a norbornane hydroxylated derivative of ID-20219 (ID-20220). Of lurasidone and its metabolites circulating in the blood, the native drug makes up 11%, the main active metabolite 4%, and the inactive carboxylic acids 24% and 11%, respectively.[4][5] Several dozen metabolites have been identified altogether.[53]:59–61
Biological half-life is given as 18 hours or 20 to 40 hours in different sources. 80% or 67% of a radiolabelled dose was recovered from the faeces, and 9% or 19% from the urine.[4][5]
History
Lurasidone was made in 2003.[54]
Lurasidone is a structural analogue of ziprasidone (Zeldox). Lurasidone shows a very close pharmacological profile and has been synthesized similarly to ziprasidone.[55]
Lurasidone is chemically similar to perospirone (also a chemical analogue of Zeldox), as well as risperidone, paliperidone and iloperidone.[56]
It has approval from the Food and Drug Administration (FDA) for treating schizophrenia since 2010 and for treating depressive episodes in adults with bipolar I disorder since 2013.[4]
Society and culture
_bottles.png.webp)
Cost
In Canada, as of 2014, lurasidone is generally more expensive than risperidone and quetiapine but less expensive than aripiprazole.[57]
In the US, because a number of doses have the same price per tablet, pill splitting has been used to decrease costs.[58] In 2019 generic versions were approved in the United States; however, they will not be available until 2023.[8][9]
Trade names
In India, this drug is available under the brand names of Atlura, Lurace, Lurafic, Luramax, Lurasid, Lurastar, Latuda, Lurata[59] and additionally as Alsiva, Emsidon, Lurakem, Luratrend, Tablura, and Unison.[60]
Regulatory approval
Lurasidone was approved in the United States for the treatment of schizophrenia in October 2010[61][62] and for the treatment of depressive episodes associated with bipolar I disorder in June 2013.[16][18][19] It received regulatory approval in the United Kingdom in September 2014. In October 2014, NHS Scotland advised use of lurasidone for schizophrenic adults who have not seen improvements with previous antipsychotics due to problems that arise from weight gain or changes in metabolic pathways when taking other medications.[63] The Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) issued a positive opinion for it in January 2014, and it was approved for medical use by the EMA in March 2014.[13] It was launched in Canada for the treatment of schizophrenia in September 2012, Health Canada giving their Summary Basis of Decision (SBD) as favourable on 15 October 2012.[64] European Commission has granted a marketing authorization for once-daily oral lurasidone for the treatment of schizophrenia in adults.[65] It is approved for use in the EU.[13]
Generic versions of Lurasidone were approved for use in the United States in January 2019.[66]
References
- "Lurasidone (Latuda) Use During Pregnancy". Drugs.com. 5 February 2020. Retrieved 12 May 2020.
- "Lurasidone Hydrochloride Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Retrieved 21 March 2019.
- "Latuda 18.5mg film-coated tablets - Summary of Product Characteristics (SmPC)". (emc). 16 January 2019. Retrieved 12 May 2020.
- "Product information Latuda (lurasidone hydrochloride)" (PDF). TGA eBusiness Services. Therapeutic Goods Administration. 16 April 2014. Retrieved 1 May 2014.
- "Latuda: EPAR – Product Information" (PDF). European Medicines Agency. 14 April 2016.
- British national formulary: BNF 76 (76 ed.). Pharmaceutical Press. 2018. pp. 393–394. ISBN 9780857113382.
- "Lurasidone (Latuda™) tablets for the treatment of schizophrenia in adults" (PDF). Retrieved 30 April 2020.
- "Generic Latuda Availability". Drugs.com. Retrieved 30 April 2020.
- Hopkins, Jared S. (19 November 2019). "Generic-Drug Approvals Soar, But Patients Still Go Without". Wall Street Journal. Retrieved 30 April 2020.
- "The Top 300 of 2020". ClinCalc. Retrieved 11 April 2020.
- "Lurasidone Hydrochloride - Drug Usage Statistics". ClinCalc. Retrieved 11 April 2020.
- "Latuda- lurasidone hydrochloride tablet, film coated". DailyMed. Retrieved 12 May 2020.
- "Latuda EPAR". European Medicines Agency (EMA). Retrieved 12 May 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - Information, National Center for Biotechnology; Pike, U. S. National Library of Medicine 8600 Rockville; MD, Bethesda; Usa, Bethesda (2014). KEY LIMITATIONS. Canadian Agency for Drugs and Technologies in Health. Retrieved 30 April 2020.
- Leucht S, Cipriani A, Spineli L, Mavridis D, Orey D, Richter F, Samara M, Barbui C, Engel RR, Geddes JR, Kissling W, Stapf MP, Lässig B, Salanti G, Davis JM (September 2013). "Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis". Lancet. 382 (9896): 951–62. doi:10.1016/S0140-6736(13)60733-3. PMID 23810019. S2CID 32085212.
- Lowes R (2013). "Lurasidone Approved for Bipolar Depression". Medscape.
- Bawa R, Scarff JR (2015). "Lurasidone: a new treatment option for bipolar depression-a review". Innovations in Clinical Neuroscience. 12 (1–2): 21–3. PMC 4382136. PMID 25852975.
- "Latuda Supplement Approval Package 1" (PDF). U.S. Food and Drug Administration (FDA). Retrieved 12 May 2020.
- "Latuda Supplement Approval Package 2" (PDF). U.S. Food and Drug Administration (FDA). Retrieved 12 May 2020.
- Young AH, McElroy SL, Bauer M, Philips N, Chang W, Olausson B, Paulsson B, Brecher M, et al. (EMBOLDEN I (Trial 001) Investigators) (February 2010). "A double-blind, placebo-controlled study of quetiapine and lithium monotherapy in adults in the acute phase of bipolar depression (EMBOLDEN I)". The Journal of Clinical Psychiatry. 71 (2): 150–62. doi:10.4088/JCP.08m04995gre. PMID 20122369.
- Suppes T, Datto C, Minkwitz M, Nordenhem A, Walker C, Darko D (February 2010). "Effectiveness of the extended release formulation of quetiapine as monotherapy for the treatment of acute bipolar depression". Journal of Affective Disorders. 121 (1–2): 106–15. doi:10.1016/j.jad.2009.10.007. PMID 19903574.
- "Corrigendum". Bipolar Disorders. 10 (3): 451. 2008. doi:10.1111/j.1399-5618.2008.00585.x.
- Thase ME (February 2008). "Quetiapine monotherapy for bipolar depression". Neuropsychiatric Disease and Treatment. 4 (1): 11–21. doi:10.2147/ndt.s1162. PMC 2515925. PMID 18728771.
- Tohen M, Vieta E, Calabrese J, Ketter TA, Sachs G, Bowden C, Mitchell PB, Centorrino F, Risser R, Baker RW, Evans AR, Beymer K, Dube S, Tollefson GD, Breier A (November 2003). "Efficacy of olanzapine and olanzapine-fluoxetine combination in the treatment of bipolar I depression". Archives of General Psychiatry. 60 (11): 1079–88. doi:10.1001/archpsyc.60.11.1079. PMID 14609883.
- Tohen M, Katagiri H, Fujikoshi S, Kanba S (July 2013). "Efficacy of olanzapine monotherapy in acute bipolar depression: a pooled analysis of controlled studies". Journal of Affective Disorders. 149 (1–3): 196–201. doi:10.1016/j.jad.2013.01.022. PMID 23485111.
- Corya SA, Perlis RH, Keck PE, Lin DY, Case MG, Williamson DJ, Tohen MF (May 2006). "A 24-week open-label extension study of olanzapine-fluoxetine combination and olanzapine monotherapy in the treatment of bipolar depression". The Journal of Clinical Psychiatry. 67 (5): 798–806. doi:10.4088/JCP.v67n0514. PMID 16841630.
- Azorin JM, Sapin C, Weiller E (February 2013). "Effect of asenapine on manic and depressive symptoms in bipolar I patients with mixed episodes: results from post hoc analyses". Journal of Affective Disorders. 145 (1): 62–9. doi:10.1016/j.jad.2012.07.013. PMID 22868059.
- Cipriani A, Barbui C, Salanti G, Rendell J, Brown R, Stockton S, Purgato M, Spineli LM, Goodwin GM, Geddes JR (October 2011). "Comparative efficacy and acceptability of antimanic drugs in acute mania: a multiple-treatments meta-analysis". Lancet. 378 (9799): 1306–15. doi:10.1016/S0140-6736(11)60873-8. PMID 21851976. S2CID 25512763.
- "Lurasidone". MedlinePlus. Retrieved 2018-09-11.
- Chiu YY, Ereshefsky L, Preskorn SH, Poola N, Loebel A (2014). "Lurasidone drug-drug interaction studies: a comprehensive review". Drug Metabolism and Drug Interactions. 29 (3): 191–202. doi:10.1515/dmdi-2014-0005. PMID 24825095.
- Pregnancy category
- ACOG Committee on Practice Bulletins--Obstetrics (April 2008). "ACOG Practice Bulletin: Clinical management guidelines for obstetrician-gynecologists number 92, April 2008 (replaces practice bulletin number 87, November 2007). Use of psychiatric medications during pregnancy and lactation". Obstetrics and Gynecology. 111 (4): 1001–20. doi:10.1097/AOG.0b013e31816fd910. PMID 18378767.
- Moore TA (April 2011). "Schizophrenia treatment guidelines in the United States". Clinical Schizophrenia & Related Psychoses. 5 (1): 40–9. doi:10.3371/CSRP.5.1.6. PMID 21459738.
- "Lurasidone Demonstrated Efficacy in Treating Patients With Schizophrenia in Pivotal Phase 3 Study" (Press release). Dainippon Sumitomo Pharma. Aug 26, 2009. Retrieved October 3, 2016.
- "Latuda: Prescribing Information". Psychotherapeutic Drugs. Archived from the original on 2011-06-28. Retrieved 2010-12-17.
- "Latuda". Drugs.com. Retrieved 2010-12-17.
- Herrmann N, Mamdani M, Lanctôt KL (June 2004). "Atypical antipsychotics and risk of cerebrovascular accidents". The American Journal of Psychiatry. 161 (6): 1113–5. doi:10.1176/appi.ajp.161.6.1113. PMID 15169702.
- Sunovion Pharmaceuticals. "Latuda Prescribing Information" (PDF). Latuda.com. Retrieved 25 March 2014.
- Meyer JM, Mao Y, Pikalov A, Cucchiaro J, Loebel A (November 2015). "Weight change during long-term treatment with lurasidone: pooled analysis of studies in patients with schizophrenia". International Clinical Psychopharmacology. 30 (6): 342–50. doi:10.1097/YIC.0000000000000091. PMC 4593468. PMID 26196189.
- Ketter TA, Sarma K, Silva R, Kroger H, Cucchiaro J, Loebel A (May 2016). "Lurasidone in the long-term treatment of patients with bipolar disorder: A 24-week open-label extension study". Depression and Anxiety. 33 (5): 424–34. doi:10.1002/da.22479. PMC 5069590. PMID 26918425. Lay summary – NEJM Journal Watch (March 14, 2016).
- Joint Formulary Committee, BMJ, ed. (March 2009). "4.2.1". British National Formulary (57 ed.). United Kingdom: Royal Pharmaceutical Society of Great Britain. p. 192. ISBN 978-0-85369-845-6.
Withdrawal of antipsychotic drugs after long-term therapy should always be gradual and closely monitored to avoid the risk of acute withdrawal syndromes or rapid relapse.
- Haddad, Peter; Haddad, Peter M.; Dursun, Serdar; Deakin, Bill (2004). Adverse Syndromes and Psychiatric Drugs: A Clinical Guide. OUP Oxford. pp. 207–216. ISBN 9780198527480.
- Moncrieff J (July 2006). "Does antipsychotic withdrawal provoke psychosis? Review of the literature on rapid onset psychosis (supersensitivity psychosis) and withdrawal-related relapse". Acta Psychiatrica Scandinavica. 114 (1): 3–13. doi:10.1111/j.1600-0447.2006.00787.x. PMID 16774655. S2CID 6267180.
- Sacchetti, Emilio; Vita, Antonio; Siracusano, Alberto; Fleischhacker, Wolfgang (2013). Adherence to Antipsychotics in Schizophrenia. Springer Science & Business Media. p. 85. ISBN 9788847026797.
- Roth BL, Driscol J. "PDSP Ki Database". Psychoactive Drug Screening Program (PDSP). University of North Carolina at Chapel Hill and the United States National Institute of Mental Health. Retrieved 14 August 2017.
- Ishibashi T, Horisawa T, Tokuda K, Ishiyama T, Ogasa M, Tagashira R, Matsumoto K, Nishikawa H, Ueda Y, Toma S, Oki H, Tanno N, Saji I, Ito A, Ohno Y, Nakamura M (July 2010). "Pharmacological profile of lurasidone, a novel antipsychotic agent with potent 5-hydroxytryptamine 7 (5-HT7) and 5-HT1A receptor activity". The Journal of Pharmacology and Experimental Therapeutics. 334 (1): 171–81. doi:10.1124/jpet.110.167346. PMID 20404009. S2CID 12893717.
- Ishiyama T, Tokuda K, Ishibashi T, Ito A, Toma S, Ohno Y (October 2007). "Lurasidone (SM-13496), a novel atypical antipsychotic drug, reverses MK-801-induced impairment of learning and memory in the rat passive-avoidance test". European Journal of Pharmacology. 572 (2–3): 160–70. doi:10.1016/j.ejphar.2007.06.058. PMID 17662268.
- Samalin L, Ben Gharbia M, Garnier M, Llorca PM (December 2014). "[Short-term efficacy and safety of lurasidone in the treatment of schizophrenia]" [Short-term efficacy and safety of lurasidone in the treatment of schizophrenia]. L'Encephale (in French). 40 (6): 507–17. doi:10.1016/j.encep.2014.10.009. PMID 25453735.
- Lincoln J, Tripathi A (2011). "Lurasidone for Schizophrenia". Current Psychiatry. 10 (1): 67–70.
- Greenberg WM, Citrome L (May 2017). "Pharmacokinetics and Pharmacodynamics of Lurasidone Hydrochloride, a Second-Generation Antipsychotic: A Systematic Review of the Published Literature". Clinical Pharmacokinetics. 56 (5): 493–503. doi:10.1007/s40262-016-0465-5. PMID 27722855. S2CID 207485482.
- Katteboina MY, Pilli NR, Mullangi R, Seelam RR, Satla SR (July 2016). "LC-MS/MS assay for the determination of lurasidone and its active metabolite, ID-14283 in human plasma and its application to a clinical pharmacokinetic study". Biomedical Chromatography. 30 (7): 1065–1074. doi:10.1002/bmc.3651. PMID 26577488.
- Caccia S, Pasina L, Nobili A (2012). "Critical appraisal of lurasidone in the management of schizophrenia". Neuropsychiatric Disease and Treatment. 8: 155–68. doi:10.2147/NDT.S18059. PMC 3346058. PMID 22570547.
- "Lurasidone pharmacology review" (PDF). Center for Drug Evaluation and Research. 30 December 2009.
- "Leading Latuda through the crowd". 1 October 2011.
- Vardanyan R, Hruby V (7 January 2016). Synthesis of Best-Seller Drugs. Academic Press. ISBN 9780124115248 – via Google Books.
- (PDF). 17 November 2016 https://web.archive.org/web/20161117142131/http://excli.de/vol13/Mauri_13102014_proof.pdf. Archived from the original (PDF) on 2016-11-17. Missing or empty
|title=(help) - Information, National Center for Biotechnology; Pike, U. S. National Library of Medicine 8600 Rockville; MD, Bethesda; Usa, Bethesda (2014). SUMMARY OF PHARMACOECONOMIC SUBMISSION. Canadian Agency for Drugs and Technologies in Health.
- Carey, H; Fondriest, M (June 2017). "Cost-Savings From an Antipsychotic Tablet-Splitting Program". P & T: A Peer-Reviewed Journal for Formulary Management. 42 (6): 384–393. PMC 5440099. PMID 28579725.
- "'Lurasidone' drug search". CIMS India. Retrieved 2018-04-21.
- "Generic Drugs (ndrugs.com)". Retrieved 2018-04-30.
- "Drug Approval Package: Latuda (lurasidone hydrochloride) Tablets NDA #200603". U.S. Food and Drug Administration (FDA). 24 December 1999. Retrieved 12 May 2020.
- "FDA approves Latuda to treat schizophrenia in adults" (Press release). U.S. Food and Drug Administration (FDA). 2010-10-28. Archived from the original on 2010-10-30. Retrieved October 29, 2010.
- "Lurasidone, 18.5mg, 37mg, 74mg film-coated tablets (Latuda) SMC No. (994/14)" (PDF). scottishmedicines.org.uk. Scottish Medicines Consortium. 2014.
- "Summary Basis of Decision (SBD) for Latuda". hc-sc.gc.ca. Health Canada. 2012.
- "European Marketing Authorization for Latuda". takeda.com. Archived from the original on 26 December 2017. Retrieved 25 November 2015.
- "Lurasidone: FDA-Approved Drugs". U.S. Food and Drug Administration (FDA). Retrieved 12 May 2020.
External links
- "Lurasidone". Drug Information Portal. U.S. National Library of Medicine.
- "FDA urges caution about withholding opioid addiction medications". U.S. Food and Drug Administration (FDA). 20 September 2017.