Macrocycle
Macrocycles are often described as molecules and ions containing twelve or more membered ring. Classical examples include the crown ethers, calixarenes, porphyrins, and cyclodextrins. Macrocycles describe a large, mature area of chemistry.[2]
Note 1: A cyclic macromolecule has no end-groups but may nevertheless be regarded as a chain.
Note 2: In the literature, the term macrocycle is sometimes used for molecules of low relative molecular mass that would not be considered macromolecules.[3]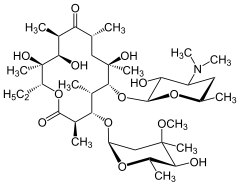
Synthesis
The formation of macrocycles by ring-closure is called macrocylization.[4] Pioneering work was reported for studies on terpenoid macrocycles.[5] The central challenge to macrocyclization is that ring-closing reactions do not favor the formation of large rings. Instead, small rings or polymers tend to form. This kinetic problem can be addressed by using high-dilution reactions, whereby intramolecular processes are favored relative to polymerizations.[6]
Some macrocyclizations are favored using template reactions. Templates are ions, molecules, surfaces etc. that bind and pre-organize compounds, guiding them toward formation of a particular ring size.[7] The crown ethers are often generated in the presence of an alkali metal cation, which organizes the condensing components by complexation.[8] An illustrative macrocyclization is the synthesis of (−)-muscone from (+)-citronellal. The 15-membered ring is generated by ring-closing metathesis.[9]
 Synthesis of muscone via RCM
Synthesis of muscone via RCM
Occurrence and applications
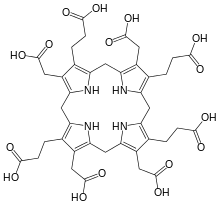
One important application are the many macrocyclic antibiotics, the macrolides, e.g. clarithromycin. Many metallocofactors are bound to macrocyclic ligands, which include porphyrins, corrins, and chlorins. These rings arise from multistep biosynthetic processes that also feature macrocycles.
Macrocycles often bind ions and facilitate ion transport across hydrophobic membranes and solvents. The macrocycle envelops the ion with a hydrophobic sheath, which facilitates phase transfer properties.[11]
Macrocycles are often bioactive and could be useful for drug delivery.[12][13]
References
- Hamilton-Miller, JM (1973). "Chemistry and Biology of the Polyene Macrolide Antibiotics". Bacteriological Reviews. 37 (2): 166–196. PMC 413810. PMID 4578757.
- Zhichang Liu; Siva Krishna Mohan Nalluria; J. Fraser Stoddart (2017). "Surveying macrocyclic chemistry: from flexible crown ethers to rigid cyclophanes". Chemical Society Reviews. 46 (9): 2459–2478. doi:10.1039/c7cs00185a. PMID 28462968.
- R. G. Jones; J. Kahovec; R. Stepto; E. S. Wilks; M. Hess; T. Kitayama; W. V. Metanomski (2008). IUPAC. Compendium of Polymer Terminology and Nomenclature, IUPAC Recommendations 2008 (the "Purple Book") (PDF). RSC Publishing, Cambridge, UK.
- François Diederich; Peter J. Stang; Rik R. Tykwinski, eds. (2008). Modern Supramolecular Chemistry: Strategies for Macrocycle Synthesis. Wiley‐VCH. doi:10.1002/9783527621484. ISBN 9783527621484.
- H. Höcker (2009). "Cyclic and Macrocyclic OrganicCompounds – a Personal Review in Honor of Professor Leopold Ružička". Cyclic and Macrocyclic Organic Compounds, Kem. Ind. 58: 73–80.
- Vicente Martí-Centelles; Mrituanjay D. Pandey; M. Isabel Burguete; Santiago V. Luis (2015). "Macrocyclization Reactions: The Importance of Conformational, Configurational, and Template-Induced Preorganization". Chem. Rev. 115 (16): 8736–8834. doi:10.1021/acs.chemrev.5b00056. PMID 26248133.
- Gerbeleu, Nicolai V.; Arion, Vladimir B.; Burgess, John (2007). Nicolai V. Gerbeleu; Vladimir B. Arion; John Burgess (eds.). Template Synthesis of Macrocyclic Compounds. Wiley‐VCH. doi:10.1002/9783527613809. ISBN 9783527613809.
- Pedersen, Charles J. (1988). "Macrocyclic Polyethers: Dibenzo-18-Crown-6 Polyether and Dicyclohexyl-18-Crown-6 Polyether". Organic Syntheses.; Collective Volume, 6, p. 395
- Kamat, V.P.; Hagiwara, H.; Katsumi, T.; Hoshi, T.; Suzuki, T.; Ando, M. (2000). "Ring Closing Metathesis Directed Synthesis of (R)-(−)-Muscone from (+)-Citronellal". Tetrahedron. 56 (26): 4397–4403. doi:10.1016/S0040-4020(00)00333-1.
- Paul R. Ortiz de Montellano (2008). "Hemes in Biology". Wiley Encyclopedia of Chemical Biology. John Wiley & Sons. doi:10.1002/9780470048672.wecb221. ISBN 978-0470048672.
- Choi, Kihang; Hamilton, Andrew D. (2003). "Macrocyclic anion receptors based on directed hydrogen bonding interactions". Coordination Chemistry Reviews. 240 (1–2): 101–110. doi:10.1016/s0010-8545(02)00305-3.
- Ermert, Philipp (2017-10-25). "Design, Properties and Recent Application of Macrocycles in Medicinal Chemistry". CHIMIA International Journal for Chemistry. 71 (10): 678–702. doi:10.2533/chimia.2017.678. PMID 29070413.
- Marsault, Eric; Peterson, Mark L. (2011-04-14). "Macrocycles Are Great Cycles: Applications, Opportunities, and Challenges of Synthetic Macrocycles in Drug Discovery". Journal of Medicinal Chemistry. 54 (7): 1961–2004. doi:10.1021/jm1012374. ISSN 0022-2623. PMID 21381769.
Further reading
- Chambron, J-C.; Dietrich-Buchecker, C.; Hemmert, C.; Khemiss, A-K.; Mitchell, D.; Sauvage, J-P.; Weiss, J. (1990). "Interlacing molecular threads on transition metals" (PDF). Pure Appl. Chem. 62 (6): 1027–34. doi:10.1351/pac199062061027.
- Iyoda, Masahiko; Yamakawa, Jun; Rahman, M. Jalilur (2011-11-04). "Conjugated Macrocycles: Concepts and Applications". Angewandte Chemie International Edition. 50 (45): 10522–10553. doi:10.1002/anie.201006198. ISSN 1521-3773. PMID 21960431.