Many-banded krait
The many-banded krait (Bungarus multicinctus), also known as the Taiwanese krait or the Chinese krait, is a venomous species of elapid snake found in much of central and southern China and Southeast Asia. The species was first described by the scientist Edward Blyth in 1861. This species has two known subspecies, the nominate Bungarus multicinctus multicinctus, and Bungarus multicinctus wanghaotingi.[3] The many-banded krait mostly inhabits marshy areas throughout its geographical distribution, though it does occur in other habitat types.
| Many-banded krait | |
|---|---|
 | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Reptilia |
| Order: | Squamata |
| Suborder: | Serpentes |
| Family: | Elapidae |
| Genus: | Bungarus |
| Species: | B. multicinctus |
| Binomial name | |
| Bungarus multicinctus | |
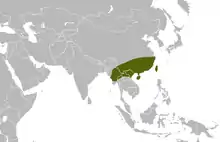 | |
| Range of the many-banded krait | |
Etymology
Since the species' description by zoologist and pharmacist Edward Blyth in 1861,[4][5] Bungarus multicinctus has been the binomial of the species. The generic name, Bungarus, is a Latinisation of Telugu baṅgāru, "krait."[6] The specific name multicinctus is derived from the Latin multi-, combining form of multus, "much, many",[7] and Latin cinctus, past participle of cingere, "to encircle"—as in a "band".[8] The full species name (Bungarus multicinctus) thus literally means "many-banded krait". The common name "krait" is from Hindi (करैत karait), which is perhaps ultimately derived from the Sanskrit word (काल kāla), which means "black".[9] It is also called as "கட்டுவிரியன்" in Tamil, a common name given to the genus Bungarus.
Taxonomy and evolution
.JPG.webp)
The kraits, as they are commonly known, belong to the family Elapidae and the genus Bungarus. The genus is endemic to the continent of Asia. They are morphologically well connected, forming a close-knit and cohesive unit. The genus has 12–13 species who are morphologically distinct from the genus Naja and the Afro-elapids according to McDowell (1987). McDowell stated "species diversity is greatest in Africa, but the Asiatic Bungarus and Ophiophagus are each so peculiar in anatomy as to suggest an ancient divergence".[10] Others, including Slowinski, believed that the kraits (Bungarus), are part of a clade that clusters with a group including the king cobra (Ophiophagus hannah) and oddly enough, with the African mambas (Dendroaspis) on the most-parsimonious tree or with Elapsoidea on the maximum-likelihood tree. This result calls into question the monophyly of cobras and underscores the uncertainty of the homology of the hood spreading behavior in cobras and mambas. The relationships of Dendroaspis, Ophiophagus, and Bungarus differed between the parsimony and likelihood analyses, suggesting that more work is necessary to resolve the relationships of these problematic taxa.[11] McDowell's findings in regard to the sister-group of Bungarus and the sea snakes (Hydrophiinae) family, propose that the kraits might just be a per-mutable clade between the elapidae "palatine-erectors" and the hydrophiinae "palatine draggers". Two genera within the subfamily Hydrophiinae in particular, support McDowell's hypothesis. The two genera are Salomonelaps and Loveridgelaps due to many shared characteristics.[12] Mao et al. (1983) showed that this species, Bungarus multicinctus was slightly distinct from the other members of its genus and was immunologically more similar to Laticauda, terrestrial Australian elapids, and true sea snakes than it is to Elapsoidea sundevalli (Sundevall's garter snake), Naja naja (Indan cobra) or two Micrurus species (New World or American coral snakes).[13] Minton (1981), Schwaner et al. and Cadle & Gorman (1981) all suggested similar things to Mao et al. (1983) based on immunological data. The many-banded krait was more similar to the Australian elapids, Laticauda and true sea snakes than they were to numerous elapids they were compared to.[14]
Description

The many-banded krait is a medium to large sized species of snake, averaging 1 to 1.5 m (3.3 to 4.9 ft) in length, with maximum lengths reaching 1.85 m (6.1 ft). Its body is slender and moderately compressed. The scales of this species are smooth and glossy, with a noticeably distinct vertebral ridge. The colour of the snake is black to dark bluish-black with approximately 21–30 white or creamy white cross bands along the entire length of its upper body. More banding is seen in longer than average sized specimens. The tail is short and pointed, that is also black in colour with alternating white cross bands, of which there are 7–11. The belly of the snake is usually white in colour, but could be an off white or creamy white.[15] The head is primarily black in colour, is broad and oval in shape, but flat and slightly distinct from the body. The eyes are small and black in colour. The pupils are black in colour, thus making them hardly noticeable as they blend in with the rest of the eyes. This species has large nostrils. The fangs are small, fixed and are located in the anterior of the upper jaw. Juveniles of this species usually have whitish blotches on the lower side of their heads.[16][17]
Scalation
Dorsal scales in 15 rows; ventral scales 200–231 in males, 198–227 in females; tail short and tapering; subcaudal scales single (undivided), 43–54 in males, 37–55 in females.[17]
Distribution and habitat
This species is found throughout Taiwan (including the Archipelagos of Matsu and Kinmen), in the central and southern regions of China (in the provinces of Hainan, Anhui, Sichuan, Guangdong, Guangxi, Hunan, Hubei, Yunnan, Guizhou, Jiangxi, Zhejiang, and Fujian), Hong Kong, Myanmar (Burma), Laos, and northern Vietnam.[15] It may also be found in Thailand.[16]

Although it can be found in elevations up to about 1,500 m (4,900 ft),[1] it is far more commonly found in humid lowland areas, and most often observed in subtropical, marshy regions of its range.[16] It is also frequently found in shrublands, woodlands, agricultural fields, and mangroves, often adjacent to water, such as rivers, streams, rice paddies, and ditches. It may also sometimes be found in villages and suburban areas.[15] It is able to survive in other habitats also.[1]
Behaviour and diet
.JPG.webp)
The snake is nocturnal, and may be more defensive at night. It is, however, a timid and placid species of snake. In the daytime, it hides under stones or in holes. The snake appears from April and retreats into hibernation in November. It is considered to be more defensive than the Banded krait (Bungarus fasciatus), thrashing about as it is handled.[15]
Unlike other Bungarus species, who are primarily snake-eaters, the many-banded krait usually feeds on fish,[18] but it is also preys on other species of snakes, including members of its own species. This species also feeds on rodents, eels, frogs, and occasionally lizards.[16]
Reproduction
There is limited information on the reproductive habits of this snake. Like many elapids, many-banded kraits are oviparous. Mating occurs between the months of August and September. Females usually deposit 3–15 eggs, although up to 20 eggs can be produced. The eggs are deposited in late spring or early summer, usually in the month of June. Eggs usually hatch about a month and a half later. The hatchlings are around 25 centimetres (9.8 in) in length.[15]
Venom and toxins
The venom of the many-banded krait consists of both pre- and postsynaptic neurotoxins (known as α-bungarotoxins and β-bungarotoxins, among others). By weight, almost half of the protein content of the venom is composed of β-bungarotoxins.[19]
The average venom yield from specimens kept on snake farms is about 4.6 mg—19.4 mg per bite.[15] The venom is highly toxic with LD50 values of 0.09 mg/kg[15]—0.108 mg/kg[20][21] SC, 0.113 mg/kg IV and 0.08 mg/kg IP on mice.[20][21] Based on several LD50 studies, the many-banded krait is among the most venomous land snakes in the world.[21]
α-Bungarotoxin is important for neuromuscular histology, it is known to bind irreversibly to receptors of the neuromuscular junction, and can be labelled with fluorescent proteins such as green fluorescent protein or the rhodamine dye tetramethylrhodamine isothiocyanate.[22]
Clinical symptoms
The local symptoms of victims bitten by the many-banded krait are usually neither serious swelling nor pain; the victims merely feel slightly itchy and numb. Systemic symptoms occur, in general, one to six hours after being bitten by this snake. Symptoms may include bilateral ptosis, diplopia, discomfort in the chest, general ache, weak feeling in limbs, ataxia, glossolysis, loss of voice, dysphagia, tunnel vision, and difficulty breathing. In case of serious bite, suppression of breathing may occur, leading to death.[15] Hyponatremia is also seen, but less commonly.[23]
Estimated mortality rates associated with untreated bites from this species vary between studies from 25–35%[16] to 70–100%.[24] During the Vietnam War, American soldiers referred to the many-banded krait as the "two-step snake," in the mistaken belief that its venom was lethal enough to kill within two steps.[25]
The many-banded krait gathered worldwide attention after a juvenile individual bit Joe Slowinski on 11 September 2001 in Myanmar. He died the following day, 29 hours after being bitten.[25]
References
- Ji, X.; Rao, D.-q. & Wang, Y. (2012). "Bungarus multicinctus". IUCN Red List of Threatened Species. 2012: e.T191957A2020937. doi:10.2305/IUCN.UK.2012-1.RLTS.T191957A2020937.en.
- "Bungarus multicinctus". Integrated Taxonomic Information System. Retrieved 4 January 2014.
- "Bungarus multicinctus". Integrated Taxonomic Information System. Retrieved 13 July 2014.
- "Bungarus". Integrated Taxonomic Information System. Retrieved 4 January 2014.
- Blyth, E. (1860). "Proceedings of the Society. Report of the Curator". The Journal of the Asiatic Society of Bengal. 29 (1): 98. Retrieved 4 January 2014.
- "Bungarum". Unabridged Dictionary. Merriam-Webster. Retrieved 13 July 2014.
- Harper, D. "multi-". The American Heritage Dictionary. Chester County Historical Society. Retrieved 5 January 2014.
- Harper, D. "Cincture". Online Etymology Dictionary. Chester County Historical Society. Retrieved 5 January 2014.
- "Krait - Bungarus". The American Heritage Dictionary. Houghton Mifflin Harcourt Publishing Company. Retrieved 5 January 2014.
- McDowell, SB (1987). Siegel. RA; Collins J; Novak SS (eds.). Snakes: Ecology and Evolutionary Biology. Toronto: Canada Macmillan Canada, Inc. pp. 3–50. ISBN 978-1-930665-15-6.
- Slowinski, JB; Knight, A; Rooney, AP (December 1997). "Inferring species trees from gene trees: A phylogenetic analysis of the Elapidae (Serpentes) based on the amino acid sequences of venom proteins". Molecular Phylogenetics and Evolution. 8 (3): 349–62. CiteSeerX 10.1.1.324.3013. doi:10.1006/mpev.1997.0434. PMID 9417893.
- McDowell, SB (August 1970). "On the status and relationships of the Solomon Island elapid snakes". Journal of Zoology. 161 (2): 145–190. doi:10.1111/j.1469-7998.1970.tb02032.x.
- Mao, SH; Chen BY; Yin FY; Guo YW (1983). "Immunotaxonomic relationships of sea snakes to terrestrial elapids". Comparative Biochemistry and Physiology A. 74 (4): 869–872. doi:10.1016/0300-9629(83)90360-2.
- Cadle, JE.; Gorman, GC. (31 July 1981). "Albumin Immunological Evidence and the Relationships of Sea Snakes". Journal of Herpetology. 15 (3): 329–334. doi:10.2307/1563437. JSTOR 1563437.
- Gopalkrishnakone ; Chou, P; LM (1996). Snakes of Medical Importance (Asia-Pacific Region). Singapore: National University of Singapore. ISBN 978-9971-62-217-6.CS1 maint: multiple names: authors list (link)
- "Bungarus multicinctus". Clinical Resource Toxinology. University of Adelaide. Archived from the original on 15 March 2016. Retrieved 6 January 2014.
- Pope, CH. (1935). The reptiles of China : turtles, crocodilians, snakes, lizards. New York: American Museum of Natural History. p. 335. LCCN 35008277.
- Greene, WH. (2000). Snakes: The Evolution of Mystery in Nature. University of California Press. p. 221. ISBN 978-0-520-22487-2.
- Ziganshin, RH; Kovalchuk, SI; Arapidi, GP; Starkov, VG; Hoang, AN; et al. (December 2015). "Quantitative proteomic analysis of Vietnamese krait venoms: Neurotoxins are the major components in Bungarus multicinctus and phospholipases A2 in Bungarus fasciatus". Toxicon. 107 (Part B): 197–209. doi:10.1016/j.toxicon.2015.08.026. PMID 26341420.
- Fry, BG. "LD50 menu (Archived)". Archived from the original on 2012-04-13.
- Séan T. & Eugene G. – Dec 1999. "LD50 (Archived)". Archived from the original on 2012-02-01.
- Anderson, MJ; Cohen, MW (March 1974). "Fluorescent staining of acetylcholine receptors in vertebrate skeletal muscle". The Journal of Physiology. 237 (2): 385–400. doi:10.1113/jphysiol.1974.sp010487. PMC 1350889. PMID 4133039.
- Hung, HT; Höjer, J; Du, NT (May 2009). "Clinical features of 60 consecutive ICU-treated patients envenomed by Bungarus multicinctus". Southeast Asian J Trop Med Public Health. 40 (3): 518–24. PMID 19842438.
- White; Meier, J;J (1995). Handbook of clinical toxicology of animal venoms and poisons. CRC Press. pp. 493–588. ISBN 978-0-8493-4489-3.CS1 maint: multiple names: authors list (link)
- James, Jamie (2008). The Snake Charmer: A Life and Death in Pursuit of Knowledge. Hyperion. p. 3. ISBN 9781401302139.
External links
 Media related to Bungarus multicinctus at Wikimedia Commons
Media related to Bungarus multicinctus at Wikimedia Commons
