Marl
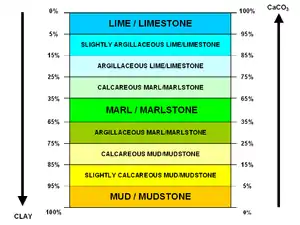
Marl or marlstone is a carbonate-rich mud or mudstone which contains variable amounts of clays and silt. The term was originally loosely applied to a variety of materials, most of which occur as loose, earthy deposits consisting chiefly of an intimate mixture of clay and calcium carbonate,[1] formed under freshwater conditions. These typically contain 35–65% clay and 65–35% carbonate.[2][3] The term is today often used to describe indurated marine deposits and lacustrine (lake) sediments which more accurately should be named 'marlstone'.[4]


Marlstone is an indurated (resists crumbling or powdering) rock of about the same composition as marl, more correctly called an earthy or impure argillaceous limestone. It has a blocky subconchoidal fracture, and is less fissile than shale.[4] The dominant carbonate mineral in most marls is calcite, but other carbonate minerals such as aragonite or dolomite may be present.[5]
The lower stratigraphic units of the chalk cliffs of Dover consist of a sequence of glauconitic marls followed by rhythmically banded limestone and marl layers. The Channel Tunnel follows these marl layers between France and the United Kingdom.[6] Upper Cretaceous cyclic sequences in Germany and marl–opal-rich Tortonian-Messinian strata in the Sorbas basin related to multiple sea drawdown have been correlated with Milankovitch orbital forcing.[7]
Marl as lacustrine sediment is common in post-glacial lake-bed sediments.[8][9][10] Chara, a macroalga also known as stonewort, thrives in shallow lakes with high pH and alkalinity, where its stems and fruiting bodies become calcified. After the alga dies, the calcified stems and fruiting bodies break down into fine carbonate particles that mingle with silt and clay to produce marl.[11] Marl ponds of the northeastern United States are often kettle ponds in areas of limestone bedrock that become poor in nutrients (oligotrophic) due to precipitation of essential phosphate. Normal pond life is unable to survive, and skeletons of freshwater mollluscs such as Sphaerium and Planorbis accumulate as part of the bottom marl.[9]
Marl has been used as a soil conditioner and acid soil neutralizing agent.[9][12] Marl from the Marlbrook Marl is used for the manufacture of cement.[13]
Economic geology
Historical use in agriculture
Marl was extensively mined in Central New Jersey as a soil conditioner in the 1800s. In 1863, the most common marl was blue marl. While the specific composition and properties of the marl varied depending on what layer it was found in, blue marl was generally composed of 38.70% silicic acid and sand, 30.67% oxide of iron, 13.91% carbonate of lime, 11.22% water, 4.47% potash, 1.21% magnesia, 1.14% phosphoric acid, and 0.31% sulphuric acid.[14]
Marl was in high demand for farms. An example of the amount of marl mined comes from a report from 1880, from Marlboro, Monmouth County, New Jersey, which reported the following tons of marl sold during the year:[15]
- OC Herbert Marl Pit – 9961 tons
- Uriah Smock Marl Pit – 4750 tons
- CM Conover Marl Pit – 760 tons
In the Centennial Exhibition report in 1877, marl is described in many different forms[16] and came from 69 marl pits in and around New Jersey. The report identified a number of agricultural marls types, including clay marl, blue marl, red marl, high bank marl, shell layer marl, under shell layer marl, sand marl, green marl, gray marl, and clayey marl.[17]
Modern agricultural and aquacultural uses
Marl continues to be used for agriculture into the 21st century, though less frequently.[18] The rate of application must be adjusted for the reduced content of calcium carbonate versus straight lime, expressed as the calcium carbonate equivalent. Because the carbonate in marl is predominantly calcium carbonate, magnesium deficiency may be seen in crops treated with marl if they are not also supplemented with magnesium.[12]
Marl has been used in Pamlico Sound to provide a suitable artificial substrate for oysters in a reef-like environment.[18]
See also
References
Citations
- Boggs 2006, p. 172.
- Pettijohn (1957), pp. 368-369.
- Blatt & Tracy 1996, p. 217.
- Pettijohn (1957), p. 410-411.
- Perri, Dominici & Critelli (2015).
- Harris 1996, p. 57.
- Krijgsman (2001).
- Murphy & Wilkinson (1980).
- Parker 2005.
- Wiik et al. (2015).
- Leeder 2011, p. 663.
- Warncke 2015.
- Arkansas_Geological Survey 2020.
- Geological Survey of New Jersey (1887).
- Geological Survey of New Jersey (1880), p. 184.
- Woll (1896), p. 295.
- New Jersey State Centennial Board (1877), p. 203.
- Morse & Smith 2011.
Bibliography
- New Jersey State Centennial Board (1877). Report of the New Jersey Commissioners on the Centennial Exhibition. Naar, Day, & Naar, printers. p. 203. Retrieved 2017-01-06.
- Geological Survey of New Jersey (1880). Annual Report of the State Geologist. p. 184. Retrieved 2017-01-06.
- Geological Survey of New Jersey (1887). Annual Report of the State Geologist. pp. 193–.
- Woll, F. W. (1896). "The Marls of Wisconsin". Thirteenth Annual Report of the Agricultural Experiment Station of the University of Wisconsin. 13. Madison, WI: Democrat Printing Company. p. 295. Retrieved 2017-01-06.
- "Marl". Arkansas Geological Survey. Retrieved 26 September 2020.
- Blatt, Harvey; Tracy, Robert J. (1996). Petrology : igneous, sedimentary, and metamorphic (2nd ed.). New York: W.H. Freeman. ISBN 0716724383.
- Boggs, Sam (2006). Principles of sedimentology and stratigraphy (4th ed.). Upper Saddle River, N.J.: Pearson Prentice Hall. p. 172. ISBN 0131547283.
- Harris, C.S.; et al., eds. (1996). Engineering Geology of the Channel Tunnel. London: Thomas Telford. p. 57. ISBN 0-7277-2045-7.
- Krijgsman, W. (2001). "Astrochronology for the Messinian Sorbas basin (SE Spain) and orbital (precessional) forcing for evaporite cyclicity" (PDF). Sedimentary Geology. 140 (1–2): 43–60. Bibcode:2001SedG..140...43K. doi:10.1016/S0037-0738(00)00171-8. hdl:1874/1632.
- Leeder, M. R. (2011). Sedimentology and sedimentary basins : from turbulence to tectonics (2nd ed.). Chichester, West Sussex, UK: Wiley-Blackwell. ISBN 9781405177832.
- Morse, David; Smith, Michael (2011). "Marl in the Coastal Plain of North Carolina: From Agriculture to Aquaculture". Geological Society of America Abstracts with Programs. 43 (2): 8. Retrieved 22 December 2020.
- Murphy, David H.; Wilkinson, Bruce H. (April 1980). "Carbonate deposition and facies distribution in a central Michigan marl lake". Sedimentology. 27 (2): 123–135. Bibcode:1980Sedim..27..123M. doi:10.1111/j.1365-3091.1980.tb01164.x. hdl:2027.42/72142.
- Parker, Alan (24 July 2005). "There's Marl in Them Thar Ponds". Northern Woodlands. Center for Northern Woodlands Education. Retrieved 25 September 2020.
- Perri, Francesco; Dominici, Rocco; Critelli, Salvatore (March 2015). "Stratigraphy, composition and provenance of argillaceous marls from the Calcare di Base Formation, Rossano Basin (northeastern Calabria)". Geological Magazine. 152 (2): 193–209. Bibcode:2015GeoM..152..193P. doi:10.1017/S0016756814000089.
- Pettijohn, F. J. (1957). Sedimentary Rocks (2nd ed.). New York: Harper & Brothers. OCLC 551748.
- Wiik, Emma; Bennion, Helen; Sayer, Carl D.; Davidson, Thomas A.; McGowan, Suzanne; Patmore, Ian R.; Clarke, Stewart J. (November 2015). "Ecological sensitivity of marl lakes to nutrient enrichment: evidence from Hawes Water, UK". Freshwater Biology. 60 (11): 2226–2247. doi:10.1111/fwb.12650.
- Warncke, Darryl (10 November 2015). "Lime for Michigan Soils". MSU Extension Agriculture. Mishigan State University. Retrieved 26 September 2020.
Further reading
- Schurrenberger, D., Russell, J. and Kerry Kelts. 2003. Classification of lacustrine sediments based on sedimentary components. Journal of Paleolimnology 29: 141–154.
External links
| Wikimedia Commons has media related to Marl. |
| Look up marl in Wiktionary, the free dictionary. |