Maurocalcine
Maurocalcine is a protein isolated from the venom of the scorpion Maurus palmatus, which belongs to the family Chactidae. It was first reported by Fajloun et al. in 2000.[1]
| Maurocalcine | |||||||
|---|---|---|---|---|---|---|---|
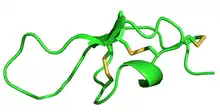 Structure of Maurocalcine, determined by NMR | |||||||
| Identifiers | |||||||
| Organism | |||||||
| Symbol | MCa | ||||||
| PDB | 1c6w | ||||||
| UniProt | P60254 | ||||||
| |||||||
It is 33 Amino acid residues in length, containing three disulphide bonds which folds into the inhibitor cystine knot motif.[2] It acts by modifying the activity of the type 1 ryanodine receptor.[1]
References
- Fajloun Z, Kharrat R, Chen L, Lecomte C, Di Luccio E, Bichet D, El Ayeb M, Rochat H, Allen PD, Pessah IN, De Waard M, Sabatier JM (March 2000). "Chemical synthesis and characterization of maurocalcine, a scorpion toxin that activates Ca(2+) release channel/ryanodine receptors". FEBS Letters. 469 (2–3): 179–85. doi:10.1016/S0014-5793(00)01239-4. PMID 10713267. S2CID 41435933.
- Mosbah A, Kharrat R, Fajloun Z, Renisio JG, Blanc E, Sabatier JM, El Ayeb M, Darbon H (August 2000). "A new fold in the scorpion toxin family, associated with an activity on a ryanodine-sensitive calcium channel". Proteins. 40 (3): 436–42. doi:10.1002/1097-0134(20000815)40:3<436::AID-PROT90>3.0.CO;2-9. PMID 10861934.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.