Membrane emulsification
Membrane emulsification (ME) is a relatively novel technique for producing all types of single and multiple emulsions for DDS (drug delivery systems), solid micro carriers for encapsulation of drug or nutrient, solder particles for surface-mount technology, mono dispersed polymer microspheres (for analytical column packing, enzyme carriers, liquid crystal display spacers, toner core particles).[1][2][3][4][5][6] Membrane emulsification was introduced by Nakashima and Shimizu in the late 1980s in Japan.[7][8]
Description
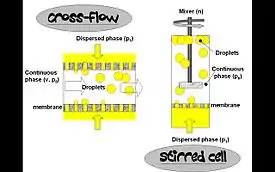
In this process, the dispersed phase is forced through the pores of a microporous membrane directly into the continuous phase. Emulsified droplets are formed and detached at the end of the pores with a drop-by-drop mechanism. The advantages of membrane emulsification over conventional emulsification processes are that it enables one to obtain very fine emulsions of controlled droplet sizes and narrow droplet size distributions. Successful emulsification can be carried out with much less consumption of emulsifier and energy, and because of the lowered shear stress effect, membrane emulsification allows the use of shear-sensitive ingredients, such as starch and proteins.[9] The membrane emulsification process is generally carried out in cross-flow (continuous or batch) mode or in a stirred cell (batch).[10][11]
A major limiting factor of ME was the low dispersed phase flux. In order to expand the industrial applications, the productivity of this method had to be increased. Some research has been aimed at solving this problem and others, such as membrane fouling.[12][13]
High dispersed phase flux has now been shown to be possible using single-pass annular gap crossflow membranes.[14]
References
- Shimizu, M.; K. Torigoe; I. Akazaki; T. Nakashima (2001). "Preparation of monodispersed solder microspheres by membrane emulsification". Proceedings of the 36th SPG Forum.
- Toorisaka, E.; H. Ono; K. Arimori; N. Kamiya; M. Goto (2003). "Hypoglycemic effect of surfactant-coated insulin solubilized in a novel solid-in-oil-in-water (S/O/W) emulsion". International Journal of Pharmaceutics. 252 (1–2): 271–274. doi:10.1016/S0378-5173(02)00674-9. PMID 12550804.
- Hosoya, K.; K. Yoshizako; K. Kimata; N. Tanaka (1997). "Chromatographic properties of uniformly sized macroporous polymer particles prepared using SPG emulsification". Chromatography - Journal of Separation and Detection Sciences. 18 (4): 226–227.
- Omi, Shinzo; Kazuyoshi Kaneko; Akira Nakayama; Ken'ichi Katami; Tetsuya Taguchi; Mamoru Iso; Masatoshi Nagai; Guang-Hui Ma (1997). "Application of porous microspheres prepared by SPG (Shirasu porous glass) emulsification as immobilizing carriers of glucoamylase (GluA)". Journal of Applied Polymer Science. 65 (13): 2655–2664. doi:10.1002/(SICI)1097-4628(19970926)65:13<2655::AID-APP7>3.0.CO;2-A.
- Higashi, S.; Shimizu M.; Nakashima T.; Iwata K.; Uchiyama F.; Taneto S.; Tamura S.; Setoguchi T. (1995). "Arterial injection chemotherapy for hepatocellular carcinoma using monodispersed poppy seed oil microdroplets containing fine aqueous vesicles of epirubicin – initial medical application of a membrane emulsification technique". Cancer. 75 (6): 1245–54. doi:10.1002/1097-0142(19950315)75:6<1245::AID-CNCR2820750606>3.0.CO;2-U. PMID 7882276.
- Huang, B.-R; Wu C.-H.; Sheu R.-F.; Ha Y.-K.; Song H.-S.; Lee H.-J.; Kim J.-H (2000). "Preparation of core particles for toner application by membrane emulsification". Colloids and Surfaces A: Physicochemical and Engineering Aspects. 162 (1): 289–293. doi:10.1016/S0927-7757(99)00235-6.
- Nakashima, T.; Shimizu M. (1986). "Porous glass from calcium alumino boro-silicate glass". Ceramics. 21: 408.
- Nakashima, T.; Shimizu M.; Kukizaki M. (1991). "Membrane Emulsification, Operation Manual". Industrial Research Institute of Miyazaki Prefecture, Miyazaki, Japan.
- Vladisavljevic, G. T.; Brösel S.; Schubert H. (2000). "Preparation of Water-in-Oil Emulsions Using Microporous Polypropylene Hollow Fibres: Conditions for Producing Small Uniform Droplets" (PDF). Chemical Papers. 54 (6a): 383–388. Archived from the original (PDF) on 2011-07-25.
- DELUCA, G; DIMAIO, F; DIRENZO, A; DRIOLI, E (1 July 2008). "Droplet detachment in cross-flow membrane emulsification: Comparison among torque- and force-based models". Chemical Engineering and Processing: Process Intensification. 47 (7): 1150–1158. doi:10.1016/j.cep.2007.03.010.
- Timgren, Anna; Trägårdh, Gun; Trägårdh, Christian (1 February 2010). "A model for drop size prediction during cross-flow emulsification". Chemical Engineering Research and Design. 88 (2): 229–238. doi:10.1016/j.cherd.2009.08.005.
- Koris, Andras; Piacentini, Emma; Vatai, Gyula; Bekassy-Molnar, Erika; Drioli, Enrico; Giorno, Lidietta (19 January 2011). "Investigation on the effects of a mechanical shear-stress modification method during cross-flow membrane emulsification". Journal of Membrane Science. 371 (1–2): 28–36. doi:10.1016/j.memsci.2011.01.005.
- Holdich, Richard G.; Dragosavac, Marijana M.; Vladisavljević, Goran T.; Kosvintsev, Serguei R. (21 April 2010). "Membrane Emulsification with Oscillating and Stationary Membranes". Industrial & Engineering Chemistry Research. 49 (8): 3810–3817. doi:10.1021/ie900531n.
- Holdich, Richard; Dragosavac, Marijana; Williams, Bruce; Trotter, Samuel (2020). "High throughput membrane emulsification using a single-pass annular flow crossflow membrane". AIChE Journal. 66 (6): e16958. doi:10.1002/aic.16958. ISSN 1547-5905.
External links
- Membrane Emulsification: Process Principles by Anneke Gijsbertsen-Abrahamse
- CFD Analysis of generation of Oil-in-water Emulsion using Rectangular Straight-through Microchannel by Kobayashi et. al.
- Membrane Emulsification video by Richard G. Holdich
- ITM-CNR, Calabria, Italy
- Micropre Technologies, UK
- SPG Technology Co, Japan