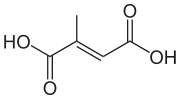
Mesaconic acid
Mesaconic acid is one of several isomeric carboxylic acids obtained from citric acid. It is a colorless solid.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
(2E)-2-Methyl-2-butenedioic acid | |
| Identifiers | |
3D model (JSmol) |
|
| 3DMet | |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.007.146 |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C5H6O4 | |
| Molar mass | 130.10 g/mol |
| Density | 1.31 g/cm3 |
| Melting point | 204 to 205 °C (399 to 401 °F; 477 to 478 K) |
| Boiling point | 250 °C (482 °F; 523 K) (decomposes) |
| Hazards | |
| GHS pictograms |  |
| GHS Signal word | Warning |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+352, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Synthesis
It is prepared from citric acid, which is first converted to itaconic anhydride by dehydration and decarboxylation. Itaconic acid anhydride is isomerized to citraconic anhydride, which is hydrolyzed and the resulting acid further isomerized under acid-catalysis to give mesaconic acid.[1]
History
This acid was studied for the first time by Jacobus H. van 't Hoff in 1874.[2] It was later shown to be produced by Clostridium tetanomorphum. Further studies showed that this organic compound is involved in the biosynthesis of vitamin B12. It is a competitive inhibitor of fumarate reduction.[3][4]
The compound has been considered as a renewable precursor to the commodity chemical methacrylic acid.[5]
References
- R. L. Shriner, S. G. Ford, L. J. Roll (1931). "Mesaconic Acid". Org. Synth. 11: 74. doi:10.15227/orgsyn.011.0074.CS1 maint: uses authors parameter (link)
- "Mesaconic acid". Mesaconic acid. Archived from the original on November 17, 2005. Retrieved September 8, 2005.
- "Barker, Horace Albert". The Stadtman Way: The Story of Two Biochemists at NIH. Office of NIH History. Retrieved December 21, 2011.
- Switzer, Robert L.; Stadtman, Earl R.; Stadtman, Thressa C. (2004). "H.A. Barker". Biographical Memoirs. National Academies Press. 84: 3–22. Retrieved December 21, 2011.
- Santosh K. Yadav, Kevin M. Schmalbach, Emre Kinaci, Joseph F. Stanzione III, Giuseppe R. Palmese (2018). "Recent advances in plant-based vinyl ester resins and reactive diluents". European Polymer Journal: 199–215. doi:10.1016/j.eurpolymj.2017.11.002.CS1 maint: uses authors parameter (link)