Metal-binding protein
Metal-binding proteins are proteins or protein domains that chelate a metal ion.[1]

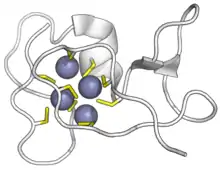
Binding of metal ions via chelation is usually achieved via histidines or cysteines. In some cases this is a necessary part of their folding and maintenance of a tertiary structure. Alternatively, a metal-binding protein may maintain its structure without the metal (apo form) and bind it as a ligand (e.g. as part of metal homeostasis). In other cases a coordinated metal cofactor is used in the active site of an enzyme to assist catalysis.
Histidine-rich metal-binding proteins
Poly-histidine tags (of six or more consecutive His residues) are utilized for protein purification by binding to columns with nickel or cobalt, with micromolar affinity.[2] Natural poly-histidine peptides, found in the venom of the viper Atheris squamigera have been shown to bind Zn(2+), Ni(2+) and Cu(2+) and affect the function of venom metalloproteases.[3] Furthermore, histidine-rich low-complexity regions are found in metal-binding and especially nickel-cobalt binding proteins.[4] These histidine-rich low complexity regions have an average length of 36 residues, of which 53% histidine, 23% aspartate, 9% glutamate.[4] Intriguingly, structured domains with metal binding properties also have very similar frequencies of these amino acids that are involved in the coordination of the metal.[5] Accordingly, it has been hypothesized that these metal-binding structured domains could have originated and evolved/optimized from metal-binding low-complexity protein regions of similar amino acid content.[4]
References
- Berg, J. M. (1990-04-25). "Zinc fingers and other metal-binding domains. Elements for interactions between macromolecules". The Journal of Biological Chemistry. 265 (12): 6513–6516. ISSN 0021-9258. PMID 2108957.
- Bornhorst, J. A.; Falke, J. J. (2000). "Purification of proteins using polyhistidine affinity tags". Methods in Enzymology. 326: 245–254. doi:10.1016/s0076-6879(00)26058-8. ISSN 0076-6879. PMC 2909483. PMID 11036646.
- Watly, Joanna; Simonovsky, Eyal; Barbosa, Nuno; Spodzieja, Marta; Wieczorek, Robert; Rodziewicz-Motowidlo, Sylwia; Miller, Yifat; Kozlowski, Henryk (2015-08-17). "African Viper Poly-His Tag Peptide Fragment Efficiently Binds Metal Ions and Is Folded into an α-Helical Structure". Inorganic Chemistry. 54 (16): 7692–7702. doi:10.1021/acs.inorgchem.5b01029. ISSN 1520-510X. PMID 26214303.
- Ntountoumi, Chrysa; Vlastaridis, Panayotis; Mossialos, Dimitris; Stathopoulos, Constantinos; Iliopoulos, Ioannis; Promponas, Vasilios; Oliver, Stephen G; Amoutzias, Grigoris D (2019-11-04). "Low complexity regions in the proteins of prokaryotes perform important functional roles and are highly conserved". Nucleic Acids Research. 47 (19): 9998–10009. doi:10.1093/nar/gkz730. ISSN 0305-1048. PMC 6821194. PMID 31504783.
- Dokmanić, Ivan; Sikić, Mile; Tomić, Sanja (March 2008). "Metals in proteins: correlation between the metal-ion type, coordination number and the amino-acid residues involved in the coordination". Acta Crystallographica. Section D, Biological Crystallography. 64 (Pt 3): 257–263. doi:10.1107/S090744490706595X. ISSN 0907-4449. PMID 18323620.