Metal carbido complex
A Metal carbido complex is an organometallic compound or coordination complex that contains the ligand C. Carbido complexes are a molecular subclass of carbides, which are prevalent. Carbido complexes represent models for intermediates in Fischer-Tropsch synthesis and related catalytic processes. They are also used as precursors for the synthesis of more complicated carbides.[1] They are analogous to metal nitrido complexes.
Terminal carbide ligands
A terminal carbido complex is RuC(PCy3)2Cl2 with a Ru-C distance of 163 pm, typical for a triple bond.[2] The complex can be obtained by metathesis of vinyl acetate to give [Ru(CH-p-C6H4Me)(PCy3)2Cl2] results in a metastable Ru(Cl2)(PCy3)2C2HOAc complex, which then decomposes to eliminate acetic acid.[3]
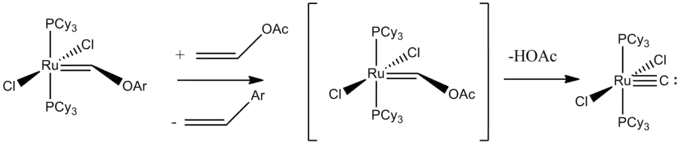
The "naked" carbido ligand is weakly basic, forming complexes with other metal centers. The C-M bond is typically found to be around 1.65 angstroms. The 13CNMR resonance values for the carbido carbons vary widely, but range from δ211-406.[4] An early example of a terminal carbido complex is Li[MoC(NR2)3], which was prepared by deprotonation of the methylidyne precursor. X-ray crystallographic analysis reveals a Mo-C distance of 172 pm.[5]
- LnM=C=M'Ln
- Metallacarbyne
- LnM≡C-M'Ln
- Polar covalent
- LnM≡C:→M'Ln
Three classes of
binuclear carbido ligands:
cumulenic, metallacarbyne,
and polar covalent.
Doubly bridging carbide ligands
Bridging carbido ligands can be subdivided into three classes, called cumulenic, metallocarbyne, and polar covalent.[6] Cumulenic compounds generally bridge two metal atoms of the same element and are symmetrical.[7] However, there are exceptions to this.[8] Metallacarbynes bridge different metal centers. Within this class, the number of bonds formed from the carbon to each metal can be determined by the valence needs of the respective metals. The polar covalent class is similar to the metallacarbyne class, but the Metal-Carbon bond with the lower bond order is more labile and can be compared to a Lewis acid adduct.
Carbido clusters
15.png.webp)
Most molecular carbido complexes are clusters, usually featuring carbide and a six-fold bridging ligand. Examples include [Rh6C(CO)15]2-,[10] and [Ru6C(CO)16]2−.[11] The iron carbonyl carbides exist not only in the encapsulated carbon ([Fe6C(CO)16]2−) but also with exposed carbon centres as in Fe5C(CO)15 and Fe4C(CO)13.[12]
Clusters without CO are also known.
6.png.webp)
See also
- Metallocarbohedryne ("met-car"), a stable cluster with formula M
8C
12 (M = Ti, Zr, V, etc.)
References
- Takemoto, Shin; Matsuzaka, H (2012). "Recent advances in the chemistry of ruthenium carbido complexes". Coordination Chemistry Reviews. 256 (5–8): 574–588. doi:10.1016/j.ccr.2011.10.025.
- Carlson, Robert G.; Gile, Melanie A.; Heppert, Joseph A.; Mason, Mark H.; Powell, Douglas R.; Vander Velde, David; Vilain, Joseph M. (2002). "The Metathesis-Facilitated Synthesis of Terminal Ruthenium Carbide Complexes: A Unique Carbon Atom Transfer Reaction". Journal of the American Chemical Society. 124 (8): 1580–1581. doi:10.1021/ja017088g. PMID 11853424.
- Caskey, Stephen (11 November 2005). "Two General Routes to Terminal Carbido Complexes". J. Am. Chem. Soc. 127 (48): 16750–16751. doi:10.1021/ja0453735. PMID 16316197.
- Hejl, A.; Trnka, T. M.; Day, M. W.; Grubbs, R. H. (2002). "Terminal ruthenium carbido complexes as sigma-donor ligands" (PDF). Chem. Commun. 21 (21): 2524–2525. doi:10.1039/b207903h.
- Peters, Jonas C.; Odom, Aaron L.; Cummins, Christopher C. (1997). "Terminal molybdenum carbide prepared by methylidyne deprotonation". Chem. Commun. (20): 1995–1996. doi:10.1039/A704251E.
- Hill, Anthony; Sharma, Willis (2012). "Heterodinuclear Bridging Carbido and Phosphocarbyne Complexes". Organometallics. 31 (7): 2538–2542. doi:10.1021/om201057c.
- Mansuy, D (1980). "New iron-poriphyrin complexes with metal-carbon bond- biological implications". Pure Appl. Chem. 52 (3): 681–690. doi:10.1351/pac198052030681.
- Solari, E; Antonijevic, S.; Gauthier, S.; Scopelliti, R.; Severin, K. (2007). "Formation of a Ruthenium μ-Carbide Complex with Acetylene as the Carbon Source". Eur. J. Inorg. Chem. 2007 (3): 367–371. doi:10.1002/ejic.200600991.
- Emile H. Braye, Lawrence F. Dahl, Walter. Hubel, Dale L. Wampler (1962). "The Preparation, Properties and Structure of the Iron Carbonyl Carbide Fe5(CO)15C". J. Am. Chem. Soc. 84 (24): 4633–4639. doi:10.1021/ja00883a004.CS1 maint: uses authors parameter (link)
- S. Martinengo, D. Strumolo, P. Chini, "Dipotassium μ6-Carbido-Nona-μ-Carbonyl-Hexacarbonylhexarhodate(2-) K2[Rh6(CO)6(μ-CO)9-μ-C]" Inorganic Syntheses 1980; 20: 212–215 doi:10.1002/9780470132517.ch48
- Cariati, Elena; Dragonetti, Claudia; Lucenti, Elena; Roberto, Dominique (2004). Tri- and Hexaruthenium Carbonyl Clusters. Inorganic Syntheses. 34. p. 210. doi:10.1002/0471653683.ch5. ISBN 9780471653677.
- Hill, Ernestine W.; Bradley, John S. (1990). Tetrairon Carbido Carbonyl Clusters. Inorganic Syntheses. 27. pp. 182–188. doi:10.1002/9780470132586.ch36. ISBN 9780470132586.