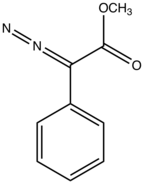
Methyl phenyldiazoacetate
Methyl phenyldiazoacetate is the organic compound with the formula C6H5C(N2)CO2Me. It is a diazo derivative of methyl phenylacetate. Colloguially referred to as "phenyldiazoacetate", it is generated and used in situ after isolation as a yellow oil.
 | |
| Identifiers | |
|---|---|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| Properties | |
| C9H8N2O2 | |
| Molar mass | 176.175 g·mol−1 |
| Appearance | yellow oil |
| alkanes | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Methyl phenyldiazoacetate and many related derivatives are precursors to donor-acceptor carbenes, which can be used for cyclopropanation or to insert into C-H bonds of organic substrates. These reactions are catalyzed by dirhodium tetraacetate or related chiral complexes.[1] Methyl phenyldiazoacetate is prepared by treating methyl phenylacetate with p-acetamidobenzenesulfonyl azide in the presence of base.[2][3]
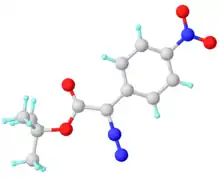
Solid state structure of t-BuO2CC(N2)C6H4NO2, a representative donor-acceptor carbene related to the title compound. Key distances: C-N = 1.329 Å, N-N = 1.121 Å.[4]
References
- Davies, H. M. L.; Morton, D. (2011). "Guiding Principles for Site Selective and Stereoselective Intermolecular C–H Functionalization by Donor/Acceptor Rhodium Carbenes". Chemical Society Reviews. 40: 1857–1869. doi:10.1039/C0CS00217H.
- Huw M. L. Davies; Wen‐hao Hu; Dong Xing. "Methyl Phenyldiazoacetate". eEROS. doi:10.1002/047084289X.rn00444.pub2.
- Selvaraj, Ramajeyam; Chintala, Srinivasa R.; Taylor, Michael T.; Fox, Joseph M. (2014). "3-Hydroxymethyl-3-phenylcyclopropene". Org. Synth. 91: 322. doi:10.15227/orgsyn.091.0322.
- Shishkov, I. V.; Rominger, F.; Hofmann, P. (2009). "Remarkably Stable Copper(I) α-Carbonyl Carbenes: Synthesis, Structure, and Mechanistic Studies of Alkene Cyclopropanation Reactions". Organometallics. 28: 1049–1059. doi:10.1021/om8007376.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.