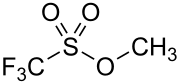
Methyl trifluoromethanesulfonate
Methyl trifluoromethanesulfonate, also commonly called methyl triflate and abbreviated MeOTf, is the organic compound with the formula CF3SO2OCH3. It is a colourless liquid which finds use in organic chemistry as a powerful methylating agent.[1] The compound is closely related to methyl fluorosulfonate (FSO2OCH3). Although there has yet to be a reported human fatality, while several cases were reported for methyl fluorosulfonate (LC50 (rat, 1 h) = 5 ppm), methyl triflate is expected to have similar toxicity based on available evidence.[1]
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Methyl trifluoromethanesulfonate | |
| Other names
Trifluoromethanesulfonic acid, methyl ester Triflic acid, methyl ester, methyl triflate | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.005.793 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C2H3F3O3S | |
| Molar mass | 164.10 g·mol−1 |
| Appearance | Colourless Liquid |
| Density | 1.496 g/mL |
| Melting point | −64 °C (−83 °F; 209 K) |
| Boiling point | 100 °C (212 °F; 373 K) |
| Hydrolyzes | |
| Hazards | |
| Main hazards | Corrosive |
| R-phrases (outdated) | R10-R34 |
| S-phrases (outdated) | S26-S36/37/39-S45 |
| Flash point | 38 °C (100 °F; 311 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Synthesis
Methyl triflate is commercially available, however it may also be prepared in the laboratory by treating dimethyl sulfate with triflic acid.[2]
- CF3SO2OH + (CH3O)2SO2 → CF3SO2OCH3 + CH3OSO2OH
Reactivity
The compound hydrolyzes violently upon contact with water:
- CF3SO2OCH3 + H2O → CF3SO2OH + CH3OH
Methylation
One ranking of methylating agents is (CH3)3O+ > CF3SO2OCH3 ≈ FSO2OCH3 > (CH3)2SO4 > CH3I.[2] Methyl triflate will alkylate many functional groups which are very poor nucleophiles such as aldehydes, amides, and nitriles. It does not methylate benzene or the bulky 2,6-di-tert-butylpyridine.[1] Its ability to methylate N-heterocycles is exploited in certain deprotection schemes.[3]
Cationic polymerization
Methyl triflate initiates the living cationic polymerization of lactide[4] and other lactones including β-propiolactone, ε-caprolactone and glycolide.[5]
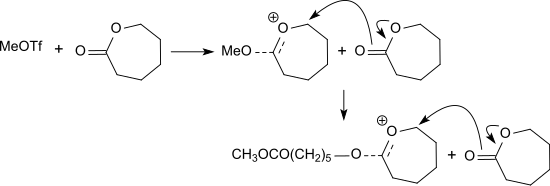
Cyclic carbonates like trimethylene carbonate and neopentylene carbonate (5,5-dimethyl-1,3-dioxan-2-one) can be polymerized to the corresponding polycarbonates.[6] 2-alkyl-2-oxazolines, for example 2-ethyl-2-oxazoline, are also polymerized to poly(2-alkyloxazoline)s.[7]
See also
- Triflate
- Magic methyl
References
- Roger W. Alder; Justin G. E. Phillips; Lijun Huang; Xuefei Huang (2005). "Methyltrifluoromethanesulfonate". Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rm266m.pub2. ISBN 0471936235.
- Stang, Peter J.; Hanack, Michael; Subramanian, L. R. (1982). "Perfluoroalkanesulfonic Esters: Methods of Preparation and Applications in Organic Chemistry". Synthesis. 1982 (2): 85–126. doi:10.1055/s-1982-29711. ISSN 0039-7881.
- Albert I. Meyers & Mark E. Flanagan (1998). "2,2′-Dimethoxy-6-formylbiphenyl". Organic Syntheses.; Collective Volume, 9, p. 258
- Rangel, Irma; Ricard, Michèle; Ricard, Alain (1994). "Polymerization of L-lactide and ε-caprolactone in the presence of methyl trifluoromethanesulfonate". Macromolecular Chemistry and Physics. 195 (9): 3095–3101. doi:10.1002/macp.1994.021950908.
- Jonté, J. Michael; Dunsing, Ruth; Kricheldorf, Hans R. (1985). "Polylactones. 4. Cationic Polymerization of Lactones by Means of Alkylsulfonates". Journal of Macromolecular Science: Part A - Chemistry. 22 (4): 495–514. doi:10.1080/00222338508056616. ISSN 0022-233X.
- Kricheldorf, Hans R.; Weegen-Schulz, Bettina; Jenssen, Jörg (1998). "Cationic polymerization of aliphatic cyclocarbonates". Macromolecular Symposia. 132 (1): 421–430. doi:10.1002/masy.19981320139.
- Glassner, Mathias; D’hooge, Dagmar R.; Young Park, Jin; Van Steenberge, Paul H.M.; Monnery, Bryn D.; Reyniers, Marie-Françoise; Hoogenboom, Richard (2015). "Systematic investigation of alkyl sulfonate initiators for the cationic ring-opening polymerization of 2-oxazolines revealing optimal combinations of monomers and initiators". European Polymer Journal. 65: 298–304. doi:10.1016/j.eurpolymj.2015.01.019. hdl:1854/LU-5924229.