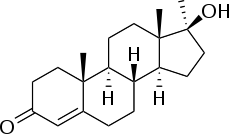
Methyltestosterone
Methyltestosterone, sold under the brand names Android, Metandren, and Testred among others, is an androgen and anabolic steroid (AAS) medication which is used in the treatment of low testosterone levels in men, delayed puberty in boys, at low doses as a component of menopausal hormone therapy for menopausal symptoms like hot flashes, osteoporosis, and low sexual desire in women, and to treat breast cancer in women.[4][5][11][12][13] It is taken by mouth or held in the cheek or under the tongue.[4][12][13][6]
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Agoviron, Android, Metandren, Oraviron, Oreton, Testovis, Testred, Virilon, others |
| Other names | RU-24400; NSC-9701; 17α-Methyltestosterone; 17α-Methylandrost-4-en-17β-ol-3-one[1][2][3] |
| AHFS/Drugs.com | Monograph |
| Pregnancy category |
|
| Routes of administration | By mouth, buccal, sublingual[4][5][6] |
| Drug class | Androgen; Anabolic steroid |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | ~70%[7] |
| Protein binding | 98%[8] |
| Metabolism | Liver |
| Elimination half-life | 150 minutes (~2.5–3 hours)[7][9] |
| Duration of action | 1–3 days[8] |
| Excretion | Urine: 90%[8] Feces: 6%[8][10] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.333 |
| Chemical and physical data | |
| Formula | C20H30O2 |
| Molar mass | 302.458 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Side effects of methyltestosterone include symptoms of masculinization like acne, increased hair growth, voice changes, and increased sexual desire.[4] It can also cause estrogenic effects like fluid retention, breast tenderness, and breast enlargement in men and liver damage.[4] The drug is a synthetic androgen and anabolic steroid and hence is an agonist of the androgen receptor (AR), the biological target of androgens like testosterone and dihydrotestosterone (DHT).[4][14] It has moderate androgenic effects and moderate anabolic effects, which make it useful for producing masculinization.[4][15]
Methyltestosterone was discovered in 1935 and was introduced for medical use in 1936.[6][16][17][18][4] It was made shortly after the discovery of testosterone and was one of the first synthetic AAS to be developed.[6][16][17] In addition to its medical use, methyltestosterone is used to improve physique and performance, although it is not as commonly used as other AAS for such purposes due to its androgenic effects, estrogenic effects, and risk of liver damage.[4] The drug is a controlled substance in many countries and so non-medical use is generally illicit.[4]
Uses
Medical
Methyltestosterone is or has been used in the treatment of delayed puberty, hypogonadism, cryptorchidism, and erectile dysfunction in males, and in low doses to treat menopausal symptoms (specifically for osteoporosis, hot flashes, and to increase libido and energy), postpartum breast pain and engorgement, and breast cancer in women.[4][5][11] It is specifically approved in the United States for the treatment of hypogonadism and delayed puberty in males and the treatment of advanced inoperable breast cancer in females.[12] It was also approved in low doses in combination with esterified estrogens for the treatment of moderate to severe vasomotor symptoms associated with menopause in women in the United States, but this formulation was discontinued and hence is no longer used.[13]
Methyltestosterone is less effective in inducing masculinization than testosterone, but is useful for maintaining established masculinization in adults.[19]
The dosages of methyltestosterone used are 10 to 50 mg/day in men for common medical uses like hypogonadism and delayed puberty as well as physique- and performance-enhancing purposes and 2.5 mg/day in women for menopausal symptoms.[4][20] Higher dosages of 50 to 200 mg/day have been used to treat women with inoperable breast cancer that has failed to respond to other therapies, although such dosages are associated with severe irreversible virilization.[4][20]
| Route | Medication | Major brand names | Form | Dosage |
|---|---|---|---|---|
| Oral | Testosteronea | – | Tablet | 400–800 mg/day (in divided doses) |
| Testosterone undecanoate | Andriol, Jatenzo | Capsule | 40–80 mg/2–4x day (with meals) | |
| Methyltestosteroneb | Android, Metandren, Testred | Tablet | 10–50 mg/day | |
| Fluoxymesteroneb | Halotestin, Ora-Testryl, Ultandren | Tablet | 5–20 mg/day | |
| Metandienoneb | Dianabol | Tablet | 5–15 mg/day | |
| Mesteroloneb | Proviron | Tablet | 25–150 mg/day | |
| Buccal | Testosterone | Striant | Tablet | 30 mg 2x/day |
| Methyltestosteroneb | Metandren, Oreton Methyl | Tablet | 5–25 mg/day | |
| Sublingual | Testosteroneb | Testoral | Tablet | 5–10 mg 1–4x/day |
| Methyltestosteroneb | Metandren, Oreton Methyl | Tablet | 10–30 mg/day | |
| Intranasal | Testosterone | Natesto | Nasal spray | 11 mg 3x/day |
| Transdermal | Testosterone | AndroGel, Testim, TestoGel | Gel | 25–125 mg/day |
| Androderm, AndroPatch, TestoPatch | Non-scrotal patch | 2.5–15 mg/day | ||
| Testoderm | Scrotal patch | 4–6 mg/day | ||
| Axiron | Axillary solution | 30–120 mg/day | ||
| Androstanolone (DHT) | Andractim | Gel | 100–250 mg/day | |
| Rectal | Testosterone | Rektandron, Testosteronb | Suppository | 40 mg 2–3x/day |
| Injection (IM or SC) | Testosterone | Andronaq, Sterotate, Virosterone | Aqueous suspension | 10–50 mg 2–3x/week |
| Testosterone propionateb | Testoviron | Oil solution | 10–50 mg 2–3x/week | |
| Testosterone enanthate | Delatestryl | Oil solution | 50–250 mg 1x/1–4 weeks | |
| Xyosted | Auto-injector | 50–100 mg 1x/week | ||
| Testosterone cypionate | Depo-Testosterone | Oil solution | 50–250 mg 1x/1–4 weeks | |
| Testosterone isobutyrate | Agovirin Depot | Aqueous suspension | 50–100 mg 1x/1–2 weeks | |
| Testosterone phenylacetateb | Perandren, Androject | Oil solution | 50–200 mg 1x/3–5 weeks | |
| Mixed testosterone esters | Sustanon 100, Sustanon 250 | Oil solution | 50–250 mg 1x/2–4 weeks | |
| Testosterone undecanoate | Aveed, Nebido | Oil solution | 750–1,000 mg 1x/10–14 weeks | |
| Testosterone buciclatea | – | Aqueous suspension | 600–1,000 mg 1x/12–20 weeks | |
| Implant | Testosterone | Testopel | Pellet | 150–1,200 mg/3–6 months |
| Notes: Men produce about 3 to 11 mg testosterone per day (mean 7 mg/day in young men). Footnotes: a = Never marketed. b = No longer used and/or no longer marketed. Sources: See template. | ||||
| Route | Medication | Major brand names | Form | Dosage |
|---|---|---|---|---|
| Oral | Testosterone undecanoate | Andriol, Jatenzo | Capsule | 40–80 mg 1x/1–2 days |
| Methyltestosterone | Metandren, Estratest | Tablet | 0.5–10 mg/day | |
| Fluoxymesterone | Halotestin | Tablet | 1–2.5 mg 1x/1–2 days | |
| Normethandronea | Ginecoside | Tablet | 5 mg/day | |
| Tibolone | Livial | Tablet | 1.25–2.5 mg/day | |
| Prasterone (DHEA)b | – | Tablet | 10–100 mg/day | |
| Sublingual | Methyltestosterone | Metandren | Tablet | 0.25 mg/day |
| Transdermal | Testosterone | Intrinsa | Patch | 150–300 μg/day |
| AndroGel | Gel, cream | 1–10 mg/day | ||
| Vaginal | Prasterone (DHEA) | Intrarosa | Insert | 6.5 mg/day |
| Injection | Testosterone propionatea | Testoviron | Oil solution | 25 mg 1x/1–2 weeks |
| Testosterone enanthate | Delatestryl, Primodian Depot | Oil solution | 25–100 mg 1x/4–6 weeks | |
| Testosterone cypionate | Depo-Testosterone, Depo-Testadiol | Oil solution | 25–100 mg 1x/4–6 weeks | |
| Testosterone isobutyratea | Femandren M, Folivirin | Aqueous suspension | 25–50 mg 1x/4–6 weeks | |
| Mixed testosterone esters | Climacterona | Oil solution | 150 mg 1x/4–8 weeks | |
| Omnadren, Sustanon | Oil solution | 50–100 mg 1x/4–6 weeks | ||
| Nandrolone decanoate | Deca-Durabolin | Oil solution | 25–50 mg 1x/6–12 weeks | |
| Prasterone enanthatea | Gynodian Depot | Oil solution | 200 mg 1x/4–6 weeks | |
| Implant | Testosterone | Testopel | Pellet | 50–100 mg 1x/3–6 months |
| Notes: Premenopausal women produce about 230 ± 70 μg testosterone per day (6.4 ± 2.0 mg testosterone per 4 weeks), with a range of 130 to 330 μg per day (3.6–9.2 mg per 4 weeks). Footnotes: a = Mostly discontinued or unavailable. b = Over-the-counter. Sources: See template. | ||||
| Route | Medication | Form | Dosage | |
|---|---|---|---|---|
| Oral | Methyltestosterone | Tablet | 30–200 mg/day | |
| Fluoxymesterone | Tablet | 10–40 mg 3x/day | ||
| Calusterone | Tablet | 40–80 mg 4x/day | ||
| Normethandrone | Tablet | 40 mg/day | ||
| Buccal | Methyltestosterone | Tablet | 25–100 mg/day | |
| Injection (IM or SC) | Testosterone propionate | Oil solution | 50–100 mg 3x/week | |
| Testosterone enanthate | Oil solution | 200–400 mg 1x/2–4 weeks | ||
| Testosterone cypionate | Oil solution | 200–400 mg 1x/2–4 weeks | ||
| Mixed testosterone esters | Oil solution | 250 mg 1x/week | ||
| Methandriol | Aqueous suspension | 100 mg 3x/week | ||
| Androstanolone (DHT) | Aqueous suspension | 300 mg 3x/week | ||
| Drostanolone propionate | Oil solution | 100 mg 1–3x/week | ||
| Metenolone enanthate | Oil solution | 400 mg 3x/week | ||
| Nandrolone decanoate | Oil solution | 50–100 mg 1x/1–3 weeks | ||
| Nandrolone phenylpropionate | Oil solution | 50–100 mg/week | ||
| Note: Dosages are not necessarily equivalent. Sources: See template. | ||||
Non-medical
Methyltestosterone is used for physique- and performance-enhancing purposes by competitive athletes, bodybuilders, and powerlifters, although it is not commonly used relative to other AAS for such purposes.[4]
Available forms
Methyltestosterone is typically used as an oral medication.[6] It is also available under the brand names Metandren and Oreton Methyl for use specifically by buccal or sublingual administration.[6][21] Methyltestosterone is available in the form of 2, 5, 10, and 25 mg oral tablets.[22][23] It was also available in combination with estrogens as esterified estrogens/methyltestosterone (0.625 mg/1.25 mg, 1.25 mg/2.5 mg) and conjugated estrogens/methyltestosterone (0.625 mg/5.0 mg, 1.25 mg/10 mg).[22]
Contraindications
Methyltestosterone should be used with caution in women and children, as it can cause irreversible virilization.[4] Due to its estrogenicity, methyltestosterone can also accelerate epiphyseal closure and thereby produce short stature in children and adolescents.[4] It can worsen symptoms in men with benign prostatic hyperplasia.[4] Methyltestosterone should not be used in men with prostate cancer, as androgens can accelerate tumor progression.[4] The drug should be used with caution in patients with pre-existing hepatotoxicity, due to its own potential for hepatotoxicity.[4]
Side effects
Adverse effects of methyltestosterone include androgenic side effects like oily skin, acne, seborrhea, increased facial/body hair growth, scalp hair loss, increased aggressiveness and sex drive, and spontaneous erections, as well as estrogenic side effects like breast tenderness, gynecomastia, fluid retention, and edema.[4][24] In women, methyltestosterone can cause partially irreversible virilization, for instance voice deepening, hirsutism, clitoromegaly, breast atrophy, and muscle hypertrophy, as well as menstrual disturbances and reversible infertility.[4][24] In men, the drug may also cause hypogonadism, testicular atrophy, and reversible infertility at sufficiently high dosages.[4][24]
Methyltestosterone can sometimes cause hepatotoxicity, for instance elevated liver enzymes, cholestatic jaundice, peliosis hepatis, hepatomas, and hepatocellular carcinoma, with extended use.[4][24][25] It can also have adverse effects on the cardiovascular system.[4] AAS like methyltestosterone stimulate erythropoiesis (red blood cell production) and increase hematocrit levels and at high dosages can cause polycythemia (overproduction of red blood cells), which can greatly increase the risk of thrombic events such as embolism and stroke.[4] With long-term treatment, AAS can increase the risk of benign prostatic hyperplasia and prostate cancer.[4] Violent and even homicidal behavior, hypomania/mania, depression, suicidality, delusions, and psychosis have all been associated with very high dosages of AAS.[26]
Interactions
Aromatase inhibitors can be used to reduce or prevent the estrogenic effects of methyltestosterone and 5α-reductase inhibitors can be used to prevent its potentiation in so-called "androgenic" tissues and thereby improve its ratio of anabolic to androgenic activity and reduce its rate of androgenic side effects.[4] Antiandrogens like bicalutamide and cyproterone acetate can block both the anabolic and androgenic effects of AAS like methyltestosterone.
Pharmacology
Pharmacodynamics
| Medication | Ratioa |
|---|---|
| Testosterone | ~1:1 |
| Androstanolone (DHT) | ~1:1 |
| Methyltestosterone | ~1:1 |
| Methandriol | ~1:1 |
| Fluoxymesterone | 1:1–1:15 |
| Metandienone | 1:1–1:8 |
| Drostanolone | 1:3–1:4 |
| Metenolone | 1:2–1:30 |
| Oxymetholone | 1:2–1:9 |
| Oxandrolone | 1:3–1:13 |
| Stanozolol | 1:1–1:30 |
| Nandrolone | 1:3–1:16 |
| Ethylestrenol | 1:2–1:19 |
| Norethandrolone | 1:1–1:20 |
| Notes: In rodents. Footnotes: a = Ratio of androgenic to anabolic activity. Sources: See template. | |
As an AAS, methyltestosterone is an agonist of the androgen receptor (AR), similarly to androgens like testosterone and dihydrotestosterone (DHT).[4][24] It is a substrate for 5α-reductase like testosterone, and so is potentiated analogously in so-called "androgenic" tissues like the skin, hair follicles, and prostate gland via transformation into the more potent AR agonist mestanolone (17α-methyl-DHT).[4][24] As such, methyltestosterone has a relatively low ratio of anabolic to androgenic activity, with a similar ratio to that of testosterone (close to 1:1), and this makes it among the most androgenic AAS.[4][24] Due to efficient aromatization into the potent and metabolism-resistant estrogen methylestradiol (17α-methylestradiol), methyltestosterone has relatively high estrogenicity and hence potential for estrogenic side effects such as gynecomastia and fluid retention.[16][27] The drug possesses negligible progestogenic activity.[4][24]
Due to its combined disadvantages of a relatively poor ratio of anabolic to androgenic activity, unusually high estrogenicity, and the potential for hepatotoxicity (as with other 17α-alkylated AAS), methyltestosterone has not been used as commonly as many other AAS either in medicine or for physique- or performance-enhancing purposes.[4]
Absorption
Methyltestosterone has dramatically improved oral bioavailability and metabolic stability relative to testosterone.[4][24] This difference is due to the C17α methyl group, which results in steric hindrance and prevents metabolism.[4][24] The oral bioavailability of methyltestosterone is about 70%, and it is well-absorbed from the gastrointestinal tract.[7] Methyltestosterone can also be taken buccally or sublingually.[4][7] Although effective orally, methyltestosterone is more effective by these non-oral routes, which are said to approximately double its bioavailability and require half the oral dosage.[4][7][21]
Circulating levels of methyltestosterone with administration of 1.25 to 2.5 mg/day oral methyltestosterone in women are in the range of 20 to 30 ng/dL.[28] For comparison to testosterone, methyltestosterone is at least as potent as an AAS.[28] However, due to the large decrease in sex hormone-binding globulin (SHBG) levels and hence increase in free unbound testosterone caused by methyltestosterone, androgenic effects may be greater than reflected merely by methyltestosterone levels.[28]
Distribution
Methyltestosterone is highly protein-bound, by approximately 98%.[8] The medication has low but significant affinity for human serum sex hormone-binding globulin (SHBG), about 25% of that of testosterone and 5% of that of DHT.[4][29]
Metabolism
The biological half-life of methyltestosterone is approximately 3 hours (range 2.5–3.5 hours).[7][10] The duration of action of methyltestosterone is said to be 1 to 3 days, and is described as relatively short among AAS.[8][30]
Excretion
Methyltestosterone is excreted 90% in the urine as conjugates and other metabolites, and 6% in feces.[8]
Chemistry
Methyltestosterone, also known as 17α-methyltestosterone or as 17α-methylandrost-4-en-17β-ol-3-one, is a synthetic, 17α-alkylated androstane steroid and a derivative of testosterone differing from it only in the presence of a methyl group at the C17α position.[1][2][4] Close synthetic relatives of methyltestosterone include metandienone (17α-methyl-δ1-testosterone) and fluoxymesterone (9α-fluoro-11β-hydroxy-17α-methyltestosterone).[4][24]
Derivatives
Methyltestosterone and ethyltestosterone (17α-ethyltestosterone) are the parent structures of all 17α-alkylated AAS. Major 17α-alkylated AAS include the testosterone derivatives fluoxymesterone, metandienone (methandrostenolone), and methyltestosterone and the DHT derivatives oxandrolone, oxymetholone, and stanozolol.[4][24]
Synthesis
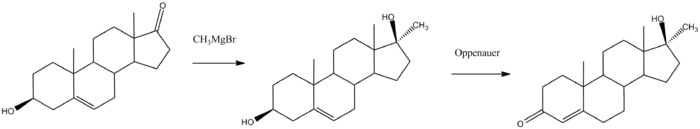
A chemical synthesis of methyltestosterone from dehydroepiandrosterone (DHEA) with methandriol as an intermediate proceeds as follows:[31][32]
History
Methyltestosterone was first synthesized in 1935 along with methandriol and mestanolone.[33][34][6][16][17] It was the second synthetic AAS to be developed, following mesterolone (1α-methyl-DHT) in 1934, and was the first 17α-alkylated AAS to be synthesized.[6][16][17] The drug was introduced for medical use in 1936.[18][4]
Society and culture

Generic names
Methyltestosterone is the INN, USAN, USP, BAN, and JAN of the drug and its generic name in English and Japanese, while méthyltestostérone is its DCF and French name and metiltestosterone is its DCIT and Italian name.[1][2][35][3] The generic name of the drug is methyltestosterone in Latin, methyltestosteron in German, and metiltestosterona in Spanish.[1][2][3] Methyltestosterone is also known by its former developmental code name NSC-9701.[35][3]
Brand names
Brand names under which methyltestosterone is or has been marketed for medical use include Afro, Agovirin, Android, Androral, Mesteron, Metandren, Methitest, Methyltestosterone, Methyl Testosterone, Oraviron, Oreton, Oreton Methyl, Testormon, Testovis, Testred, and Virilon, among others.[1][2][3][36]
With an estrogen
Methyltestosterone is available at a low-dose in combination with esterified estrogens for the treatment of menopausal symptoms like hot flashes in women under the brand names Covaryx, Essian, Estratest, Menogen, and Syntest.[4][37]
Availability
United States
Although it is not commonly used, methyltestosterone is one of the few AAS that remains available for medical use in the United States.[4][36] The others are testosterone, testosterone cypionate, testosterone enanthate, testosterone undecanoate, oxandrolone, oxymetholone, and fluoxymesterone.[36]
Other countries
Methyltestosterone has also been marketed in many other countries throughout the world.[1][2][3][4][38][39]
Legal status
Methyltestosterone, along with other AAS, is a schedule III controlled substance in the United States under the Controlled Substances Act and a schedule IV controlled substance in Canada under the Controlled Drugs and Substances Act.[40][41]
References
- J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 653–. ISBN 978-1-4757-2085-3.
- Index Nominum 2000: International Drug Directory. Taylor & Francis. 2000. pp. 676–. ISBN 978-3-88763-075-1.
- "Methyltestosterone".
- William Llewellyn (2009). Anabolics. Molecular Nutrition Llc. pp. 16, 19, 22, 27, 30, 36, 39, 42, 46, 291–293. ISBN 978-0967930473.
- Manuchair Ebadi (31 October 2007). Desk Reference of Clinical Pharmacology, Second Edition. CRC Press. pp. 434–. ISBN 978-1-4200-4744-8.
- Alexandre Hohl (30 March 2017). Testosterone: From Basic to Clinical Aspects. Springer. pp. 204–205. ISBN 978-3-319-46086-4.
- Thomas L. Lemke; David A. Williams (24 January 2012). Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. pp. 1360–. ISBN 978-1-60913-345-0.
- Teri Moser Woo; Marylou V Robinson (3 August 2015). Pharmacotherapeutics For Advanced Practice Nurse Prescribers. F.A. Davis. pp. 618–. ISBN 978-0-8036-4581-3.
- Behre, H.M.; Wang, C.; Handelsman, D.J.; Nieschlag, E.; Nieschlag, E.; Behre, H. M.; Nieschlag, S. (2004). "Pharmacology of testosterone preparations". Testosterone. pp. 405–444. doi:10.1017/CBO9780511545221.015. ISBN 9780511545221.
- Kourosh Saeb-Parsy (18 June 1999). Instant Pharmacology. John Wiley & Sons. pp. 260–. ISBN 978-0-471-97639-4.
- John A. Yagiela; Frank J. Dowd; Bart Johnson; Angelo Mariotti, Enid A. Neidle (19 March 2010). Pharmacology and Therapeutics for Dentistry - E-Book. Elsevier Health Sciences. pp. 569–. ISBN 978-0-323-07824-5.
- https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/083976s031lbl.pdf
- "DailyMed - ESTERIFIED ESTROGENS AND METHYLTESTOSTERONE - esterified estrogens and methyltestosterone tablet, film coated".
- Kicman AT (2008). "Pharmacology of anabolic steroids". Br. J. Pharmacol. 154 (3): 502–21. doi:10.1038/bjp.2008.165. PMC 2439524. PMID 18500378.
- Charles D. Kochakian (6 December 2012). Anabolic-Androgenic Steroids. Springer Science & Business Media. pp. 13, 401, 454. ISBN 978-3-642-66353-6.
- Detlef Thieme; Peter Hemmersbach (18 December 2009). Doping in Sports. Springer Science & Business Media. pp. 101, 470. ISBN 978-3-540-79088-4.
- Shahidi NT (2001). "A review of the chemistry, biological action, and clinical applications of anabolic-androgenic steroids". Clin Ther. 23 (9): 1355–90. doi:10.1016/s0149-2918(01)80114-4. PMID 11589254.
- N.A.R.D. journal. National Association of Retail Druggists. July 1956.
- John A. Thomas; Edward J. Keenan (6 December 2012). Principles of Endocrine Pharmacology. Springer Science & Business Media. pp. 125–. ISBN 978-1-4684-5036-1.
- http://www.micromedexsolutions.com/micromedex2%5B%5D
- American Medical Association. Division of Drugs; American Society for Clinical Pharmacology and Therapeutics (1983). "Androgens and Anabolic Steroids". AMA Drug Evaluations. American Medical Association. pp. 913–930. ISBN 978-0-89970-160-8.
- Jacques Lorrain (1994). Comprehensive Management of Menopause. Springer Science & Business Media. pp. 301–. ISBN 978-0-387-97972-4.
- Heinrich Kahr (8 March 2013). Konservative Therapie der Frauenkrankheiten: Anzeigen, Grenzen und Methoden Einschliesslich der Rezeptur. Springer-Verlag. pp. 21–. ISBN 978-3-7091-5694-0.
- Kicman, A T (2008). "Pharmacology of anabolic steroids". British Journal of Pharmacology. 154 (3): 502–521. doi:10.1038/bjp.2008.165. PMC 2439524. PMID 18500378.
- Jeffrey K. Aronson (21 February 2009). Meyler's Side Effects of Endocrine and Metabolic Drugs. Elsevier. pp. 141–. ISBN 978-0-08-093292-7.
- Benjamin J. Sadock; Virginia A. Sadock (26 December 2011). Kaplan and Sadock's Synopsis of Psychiatry: Behavioral Sciences/Clinical Psychiatry. Lippincott Williams & Wilkins. ISBN 978-1-4511-7861-6.
- Andrea R. Genazzani (17 January 2006). Postmenopausal Osteoporosis: Hormones & Other Therapies. Taylor & Francis US. pp. 243–. ISBN 978-1-84214-311-7.
- Lobo, Rogerio A. (2001). "Androgens in Postmenopausal Women: Production, Possible Role, and Replacement Options". Obstetric and Gynecologic Survey. 56 (6): 361–376. doi:10.1097/00006254-200106000-00022. ISSN 0029-7828. PMID 11466487. S2CID 9872335.
- Saartok T, Dahlberg E, Gustafsson JA (1984). "Relative binding affinity of anabolic-androgenic steroids: comparison of the binding to the androgen receptors in skeletal muscle and in prostate, as well as to sex hormone-binding globulin". Endocrinology. 114 (6): 2100–6. doi:10.1210/endo-114-6-2100. PMID 6539197.
- Lynn Crespo; Lynn Wecker; George Dunaway; Carl Faingold, Stephanie Watts (1 April 2009). Brody's Human Pharmacology - E-Book. Elsevier Health Sciences. pp. 469–. ISBN 978-0-323-07575-6.
- Daniel Lednicer (4 March 2009). Strategies for Organic Drug Synthesis and Design. John Wiley & Sons. pp. 144–. ISBN 978-0-470-39959-0.
- Algar (2010). Textbook Of Medicinal Chemistry. Elsevier Health Sciences. pp. 212–. ISBN 978-81-312-2190-7.
- Schänzer W (1996). "Metabolism of anabolic androgenic steroids". Clin. Chem. 42 (7): 1001–20. doi:10.1093/clinchem/42.7.1001. PMID 8674183.
- Ruzicka, L.; Goldberg, M. W.; Rosenberg, H. R. (1935). "Sexualhormone X. Herstellung des 17-Methyl-testosterons und anderer Androsten- und Androstanderivate. Zusammenhänge zwischen chemischer Konstitution und männlicher Hormonwirkung". Helvetica Chimica Acta. 18 (1): 1487–1498. doi:10.1002/hlca.193501801203. ISSN 0018-019X.
- I.K. Morton; Judith M. Hall (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 179–. ISBN 978-94-011-4439-1.
- "Drugs@FDA: FDA Approved Drug Products". United States Food and Drug Administration. Retrieved 28 June 2017.
- "Esterified Estrogens and Methyltestosterone (Oral Route) Description and Brand Names - Mayo Clinic".
- Muller (19 June 1998). European Drug Index: European Drug Registrations, Fourth Edition. CRC Press. pp. 36, 400. ISBN 978-3-7692-2114-5.
- William Andrew Publishing (22 October 2013). Pharmaceutical Manufacturing Encyclopedia. Elsevier. pp. 2109–. ISBN 978-0-8155-1856-3.
- Steven B. Karch (21 December 2006). Drug Abuse Handbook, Second Edition. CRC Press. pp. 30–. ISBN 978-1-4200-0346-8.
- Linda Lane Lilley; Julie S. Snyder; Shelly Rainforth Collins (5 August 2016). Pharmacology for Canadian Health Care Practice. Elsevier Health Sciences. pp. 50–. ISBN 978-1-77172-066-3.
Further reading
- Phillips EH, Ryan S, Ferrari R, Green C (2003). "Estratest and Estratest HS (esterified estrogens and methyltestosterone) therapy: a summary of safety surveillance data, January 1989 to August 2002". Clin Ther. 25 (12): 3027–43. doi:10.1016/s0149-2918(03)90090-7. PMID 14749144.
- Kabat GC, Kamensky V, Heo M, Bea JW, Hou L, Lane DS, Liu S, Qi L, Simon MS, Wactawski-Wende J, Rohan TE (2014). "Combined conjugated esterified estrogen plus methyltestosterone supplementation and risk of breast cancer in postmenopausal women". Maturitas. 79 (1): 70–6. doi:10.1016/j.maturitas.2014.06.006. PMID 25011395.
- El-Desoky el-SI, Reyad M, Afsah EM, Dawidar AA (2016). "Synthesis and chemical reactions of the steroidal hormone 17α-methyltestosterone". Steroids. 105: 68–95. doi:10.1016/j.steroids.2015.11.004. PMID 26639430. S2CID 32620483.