Milas hydroxylation
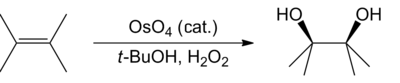
The Milas hydroxylation is an organic reaction converting an alkene to a vicinal diol, and was developed by Nicholas A. Milas in the 1930s.[1][2] The cis-diol is formed by reaction of alkenes with hydrogen peroxide and either ultraviolet light or a catalytic osmium tetroxide,[3] vanadium pentoxide, or chromium trioxide.[4]
| Milas hydroxylation | |
|---|---|
| Named after | Nicholas Milas |
| Reaction type | Addition reaction |
The reaction has been superseded in synthetic chemistry by the Upjohn dihydroxylation and later by the Sharpless asymmetric dihydroxylation.
Mechanism
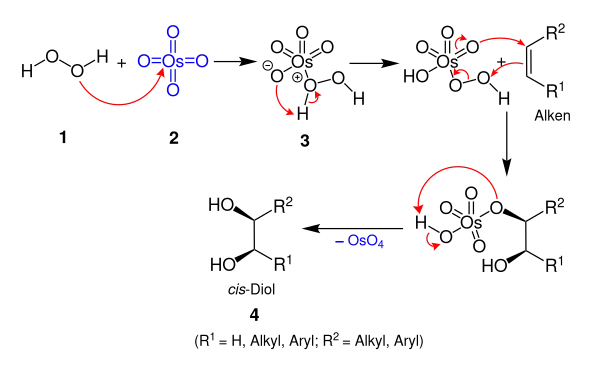
The proposed mechanism for the Milas hydroxylation involves the initial combination of hydrogen peroxide and the osmium tetroxide catalyst to form an intermediate, which then adds to the alkene, followed by a cleavage that forms the product and regenerates the OsO4.[2]
References
- Milas, Nicholas A.; Sussman, Sidney (July 1936). "The Hydroxylation of the Double Bond". Journal of the American Chemical Society. 58 (7): 1302–1304. doi:10.1021/ja01298a065.
- "Milas Hydroxylation". Comprehensive Organic Name Reactions and Reagents. 437: 1948–1951. 2010. doi:10.1002/9780470638859.conrr437. ISBN 9780470638859.
- Milas, Nicholas A.; Trepagnier, Joseph H.; Nolan, John T.; Iliopulos, Miltiadis I. (September 1959). "A Study of the Hydroxylation of Olefins and the Reaction of Osmium Tetroxide with 1,2-Glycols". Journal of the American Chemical Society. 81 (17): 4730–4733. doi:10.1021/ja01526a070.
- Milas, Nicholas A. (November 1937). "The Hydroxylation of Unsaturated Substances. III. The Use of Vanadium Pentoxide and Chromium Trioxide as Catalysts of Hydroxylation". Journal of the American Chemical Society. 59 (11): 2342–2344. doi:10.1021/ja01290a072.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.