Monohydrogen phosphate
Monohydrogen phosphate is the inorganic ion with the formula [HPO4]2-. Its formula can also be written as [PO3(OH)]2-, which shows the presence of a O-H bond. Together with dihydrogen phosphate, monohydrogen phosphate occurs widely in natural systems. Their salts are used in fertilizers and in cooking.[1] Most monohydrogenphosphate salts are colorless, water soluble, and nontoxic.
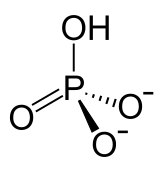
Monohydrogen phosphate anion.
Acid-base equilibria
Monohydrogenphosphate is an intermediate in the multistep conversion of phosphoric acid to phosphate:
| Equilibrium | Disassociation constant, pKa[2] |
|---|---|
| H3PO4 ⇌ H 2PO− 4 + H+ |
pKa1 = 2.14[lower-alpha 1] |
| H 2PO− 4 ⇌ HPO2− 4 + H+ |
pKa2 = 7.20 |
| HPO2− 4 ⇌ PO3− 4 + H+ |
pKa3 = 12.37 |
- Values are at 25 °C and 0 ionic strength.
Examples
- Diammonium phosphate, (NH4)2HPO4
- Disodium phosphate, Na2HPO4, with varying amounts of water of hydration
References
- Schrödter, Klaus; Bettermann, Gerhard; Staffel, Thomas; Wahl, Friedrich; Klein, Thomas; Hofmann, Thomas (2008). "Phosphoric Acid and Phosphates". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a19_465.pub3.
- Powell, Kipton J.; Brown, Paul L.; Byrne, Robert H.; Gajda, Tamás; Hefter, Glenn; Sjöberg, Staffan; Wanner, Hans (2005). "Chemical speciation of environmentally significant heavy metals with inorganic ligands. Part 1: The Hg2+, Cl−, OH−, CO2−
3, SO2−
4, and PO3−
4 aqueous systems". Pure Appl. Chem. 77 (4): 739–800. doi:10.1351/pac200577040739.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.