Nuclear RNase P
In molecular biology, nuclear ribonuclease P (RNase P) is a ubiquitous endoribonuclease, found in archaea, bacteria and eukarya as well as chloroplasts and mitochondria. Its best characterised enzyme activity is the generation of mature 5′-ends of tRNAs by cleaving the 5′-leader elements of precursor-tRNAs. Cellular RNase Ps are ribonucleoproteins. The RNA from bacterial RNase P retains its catalytic activity in the absence of the protein subunit, i.e. it is a ribozyme. Similarly, archaeal RNase P RNA has been shown to be weakly catalytically active in the absence of its respective protein cofactors.[1] Isolated eukaryotic RNase P RNA has not been shown to retain its catalytic function, but is still essential for the catalytic activity of the holoenzyme. Although the archaeal and eukaryotic holoenzymes have a much greater protein content than the bacterial ones, the RNA cores from all three lineages are homologous—the helices corresponding to P1, P2, P3, P4, and P10/11 are common to all cellular RNase P RNAs. Yet there is considerable sequence variation, particularly among the eukaryotic RNAs.
| Nuclear RNase P | |
|---|---|
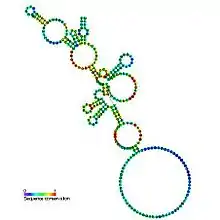 Predicted secondary structure and sequence conservation of RNaseP_nuc | |
| Identifiers | |
| Symbol | RNaseP_nuc |
| Rfam | RF00009 |
| Other data | |
| RNA type | Gene; ribozyme |
| Domain(s) | Eukaryota; Bacteria; Archaea |
| GO | GO:0008033 GO:0004526 GO:0030677 |
| SO | SO:0000386 |
| PDB structures | PDBe |
References
- Tsai, H.Y.; Pulukkunat, D.K.; Woznick, W.K.; Gopalan, V. (2006). "Functional reconstitution and characterization of Pyrococcus furiosus RNase P". PNAS. 103: 16147–16152. doi:10.1073/pnas.0608000103.
Further reading
- Frank DN, Pace NR (1998). "Ribonuclease P: unity and diversity in a tRNA processing ribozyme". Annual Review of Biochemistry. 67: 153–180. doi:10.1146/annurev.biochem.67.1.153. PMID 9759486.
- Brown JW (January 1999). "The Ribonuclease P Database". Nucleic Acids Research. 27 (1): 314. doi:10.1093/nar/27.1.314. PMC 148169. PMID 9847214.
- Frank DN, Adamidi C, Ehringer MA, Pitulle C, Pace NR (December 2000). "Phylogenetic-comparative analysis of the eukaryal ribonuclease P RNA". RNA. 6 (12): 1895–1904. doi:10.1017/S1355838200001461. PMC 1370057. PMID 11142387.
- Xiao S, Scott F, Fierke CA, Engelke DR (2002). "Eukaryotic ribonuclease P: a plurality of ribonucleoprotein enzymes". Annual Review of Biochemistry. 71: 165–189. doi:10.1146/annurev.biochem.71.110601.135352. PMC 3759807. PMID 12045094.
- Marquez SM, Harris JK, Kelley ST, Brown JW, Dawson SC, Roberts EC, Pace NR (May 2005). "Structural implications of novel diversity in eucaryal RNase P RNA". RNA. 11 (5): 739–751. doi:10.1261/rna.7211705. PMC 1370759. PMID 15811915.