Octadecene
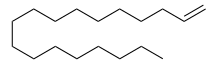
Octadecene is a long-chain hydrocarbon and an alkene with the molecular formula C18H36. There are multiple structural isomers of octadecene, depending on the position of the double bond. 1-Octadecene, an alpha-olefin, is a relatively inexpensive solvent, with a boiling point of 315 °C.[1] It is compatible with oleic acid.
 | |
| Names | |
|---|---|
| IUPAC name
Octadec-1-ene | |
| Other names
alpha-Octadecene; Octadecylene; alpha-Olefin C18; n-1-Octadecene | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.003.648 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C18H36 | |
| Molar mass | 252.486 g·mol−1 |
| Appearance | Clear liquid[1] |
| Density | 0.789 g/mL[2] |
| Melting point | 14 to 16 °C (57 to 61 °F; 287 to 289 K)[2] 17 to 18 °C[1] |
| Boiling point | 315 °C (599 °F; 588 K)[1] |
| Insoluble[1] | |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Flash point | 155 °C (311 °F; 428 K)[1] |
| 250 °C (482 °F; 523 K)[2] | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Octadecene is used in the synthesis of colloidal quantum dots, but it can possibly be replaced by heat transfer fluids such as Dowtherm A or Therminol 66 in this process.[3]
See also
References
- 1-Octadecene fact sheet ChemicalLand21
- 1-Octadecene at Sigma-Aldrich
- Asokan, Subashini; Krueger, Karl M; Alkhawaldeh, Ammar; Carreon, Alessandra R; Mu, Zuze; Colvin, Vicki L; Mantzaris, Nikos V; Wong, Michael S (2005). "The use of heat transfer fluids in the synthesis of high-quality CdSe quantum dots, core/shell quantum dots, and quantum rods". Nanotechnology. 16 (10): 2000–11. doi:10.1088/0957-4484/16/10/004. PMID 20817962.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.
