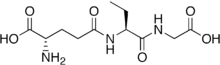
Ophthalmic acid
Ophthalmic acid, also known as ophthalmate (chemically L-γ-glutamyl-L-α-aminobutyrylglycine), is a tripeptide analog of glutathione in which the cysteine group is replaced by L-2-aminobutyrate. It was first discovered and isolated from calf lens.[2]
 | |
| Names | |
|---|---|
| IUPAC name
(N-(L-γ-Glutamyl)-(2S)-2-aminobutyryl)glycine | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| MeSH | ophthalmic+acid |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C11H19N3O6 | |
| Molar mass | 289.288 g·mol−1 |
| Appearance | Colorless crystals |
| Related compounds | |
Related alkanoic acids |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Biosynthesis
Recent studies have shown that the ophthalmate can be biologically synthesized from 2-amino butyric acid through consecutive reactions with gamma-glutamylcysteine synthetase and glutathione synthetase. So the ophthalmic acid could be used as a biomarker in oxidative stress where the depletion of glutathione takes place.[3]
See also
- Aminobutyrate
- Glutathione
- Glutathione synthetase deficiency
- Oxidative stress
References
- Ophthalmic acid
- Waley SG; Biochem. J. 64, 715 (1956)
- Tomoyoshi Soga; Richard Baran; Makoto Suematsu; Yuki Ueno; Satsuki Ikeda; Tadayuki Sakurakawa; Yuji Kakazu; Takamasa Ishikawa; Martin Robert; Takaaki Nishioka & Masaru Tomita (June 2006). "Differential Metabolomics Reveals Ophthalmic Acid as an Oxidative Stress Biomarker Indicating Hepatic Glutathione Consumption". Journal of Biological Chemistry. 281 (24): 16768–16776. doi:10.1074/jbc.M601876200. PMID 16608839.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.