Osazone
Osazones are a class of carbohydrate derivatives found in organic chemistry formed when reducing sugars are reacted with excess of phenylhydrazine at boiling temperatures.[1][2]

Ball-and-stick model of glucosazone
Formation
Osazone formation was developed by Emil Fischer,[3] who used the reaction as a test to identify monosaccharides.
The formation of a pair of hydrazone functionalities involves both oxidation and condensation reactions.[4] Since the reaction requires a free carbonyl group, only "reducing sugars" participate. Sucrose, which is nonreducing, doess not form an osazone.
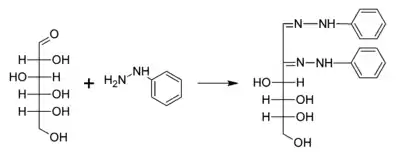 A typical reaction showing the formation of an osazone. D-glucose reacts with phenylhydrazine to give glucosazone. The same product is obtained from fructose and mannose.
A typical reaction showing the formation of an osazone. D-glucose reacts with phenylhydrazine to give glucosazone. The same product is obtained from fructose and mannose.
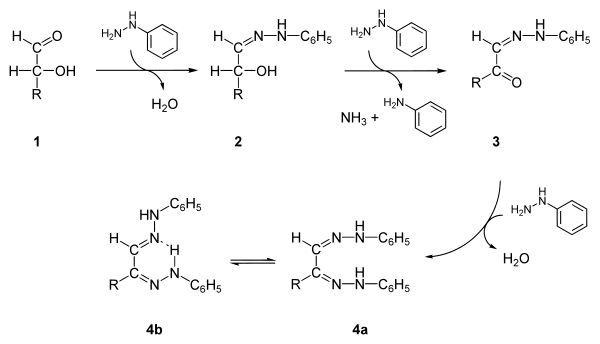 General steps in osazone formation
General steps in osazone formation
Appearance
Osazones are highly coloured and crystalline compounds. Osazones are readily distinguished.[5]
Historic references
- Fischer, Emil (1908). "Schmelzpunkt des Phenylhydrazins und einiger Osazone". Berichte der Deutschen Chemischen Gesellschaft. 41: 73–77. doi:10.1002/cber.19080410120.
- Fischer, Emil (1894). "Ueber einige Osazone und Hydrazone der Zuckergruppe". Berichte der Deutschen Chemischen Gesellschaft. 27 (2): 2486–2492. doi:10.1002/cber.189402702249.
- Barry, VINCENT C.; Mitchell, PW (1955). "Mechanism of Osazone Formation". Nature. 175 (4448): 220. Bibcode:1955Natur.175..220B. doi:10.1038/175220a0. PMID 13235861.
References
- . doi:10.1016/S0065-2318(00)55006-9. Cite journal requires
|journal=(help); Missing or empty|title=(help) - Mester, L.; El Khadem, H.; Horton, D. (1970). "Structure of saccharide osazones". Journal of the Chemical Society C: Organic (18): 2567. doi:10.1039/J39700002567.
- Helferich, B. (1953). "Emil Fischer zum 100. Geburtstag". Angewandte Chemie. 65 (2): 45–52. doi:10.1002/ange.19530650202.
- Ramakrishnan, S. (2004). Textbook of Medical Biochemistry. Orient Blackswan. ISBN 9788125020714.
- Gupta, Anil (2019). "Carbohydrates". Comprehensive Biochemistry for Dentistry. Singapore: Springer. pp. 108–110. ISBN 978-981-13-1035-5.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.