Oxycodone
Oxycodone, sold under the brand name OxyContin (which is the extended release form) among others, is an opioid medication used for treatment of moderate to severe pain, and a common drug of abuse.[12][13] It is usually taken by mouth, and is available in immediate-release and controlled-release formulations.[12] Onset of pain relief typically begins within fifteen minutes and lasts for up to six hours with the immediate-release formulation.[12] In the United Kingdom, it is available by injection.[14] Combination products are also available with paracetamol (acetaminophen), ibuprofen, naloxone, and aspirin.[12]
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | ɒksɪˈkəʊdəʊn |
| Trade names | OxyContin, Endone, others |
| Other names | Eukodal, eucodal; dihydrohydroxycodeinone, 7,8-dihydro-14-hydroxycodeinone, 6-deoxy-7,8-dihydro-14-hydroxy-3-O-methyl-6-oxomorphine[1] |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682132 |
| License data | |
| Pregnancy category |
|
| Dependence liability | High[3] |
| Routes of administration | By mouth, sublingual, intramuscular, intravenous, intranasal, subcutaneous, transdermal, rectal, epidural[4] |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | By mouth: 60–87%[5][6] |
| Protein binding | 45%[5] |
| Metabolism | Liver: mainly CYP3A, and, to a much lesser extent, CYP2D6 (~5%);[5] 95% metabolized (i.e., 5% excreted unchanged)[7] |
| Metabolites | • Noroxycodone (25%) [8][7] • Noroxymorphone (15%, free and conjugated)[8][7] • Oxymorphone (11%, conjugated)[8][7] • Others (e.g., minor metabolites)[7] |
| Onset of action | IR: 10–30 minutes[6][7] CR: 1 hour[9] |
| Elimination half-life | By mouth (IR): 2–3 hrs (same t1/2 for all ROAs)[7][6] By mouth (CR): 4.5 hrs[10] |
| Duration of action | By mouth (IR): 3–6 hrs[7] By mouth (CR): 10–12 hrs[11] |
| Excretion | Urine (83%)[5] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.874 |
| Chemical and physical data | |
| Formula | C18H21NO4 |
| Molar mass | 315.369 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 219 °C (426 °F) |
| Solubility in water | 166 (HCl) |
| |
| |
| (verify) | |
Common side effects include euphoria, constipation, nausea, vomiting, loss of appetite, drowsiness, dizziness, itching, dry mouth, and sweating.[12] Severe side effects may include addiction, dependence, hallucinations, respiratory depression (a reduction in breathing), bradycardia, and low blood pressure.[12] Those allergic to codeine may also be allergic to oxycodone.[12] Use of oxycodone in early pregnancy appears relatively safe.[12] Opioid withdrawal may occur if rapidly stopped.[12] Oxycodone acts by activating the μ-opioid receptor.[15] When taken by mouth, it has roughly 1.5 times the effect of the equivalent amount of morphine.[16]
Oxycodone was first made in Germany in 1916 from thebaine.[17] It is available as a generic medication.[12] In 2017, it was the 52nd most commonly prescribed medication in the United States, with more than fifteen million prescriptions.[18][19] A number of abuse-deterrent formulations are available, such as in combination with naloxone.[13][20]
Medical uses
Oxycodone is used for managing moderate to severe acute or chronic pain when other treatments are not sufficient.[12] It may improve quality of life in certain types of pain.[21] It is unclear if use in chronic pain results in improved quality of life or ongoing pain relief.[12]
Oxycodone is available as controlled-release tablet, intended to be taken every 12 hours.[22] A July 1996 study independent of Purdue Pharma, the drug's originator, found the controlled-release formulation had a variable duration of action ranging from 10 to 12 hours.[11] A 2006 review found that controlled-release oxycodone is comparable to immediate-release oxycodone, morphine, and hydromorphone in management of moderate to severe cancer pain, with fewer side effects than morphine. The author concluded that the controlled-release form is a valid alternative to morphine and a first-line treatment for cancer pain.[22] In 2014, the European Association for Palliative Care recommended oxycodone by mouth as a second-line alternative to morphine by mouth for cancer pain.[23]
In the U.S., extended-release oxycodone is approved for use in children as young as eleven years old. The approved uses is for relief of cancer pain, trauma pain, or pain due to major surgery, in children already treated with opioids, who can tolerate at least 20 mg per day of oxycodone; this provides an alternative to Duragesic (fentanyl), the only other extended-release opioid analgesic approved for children.[24]
Available forms
.jpg.webp)
.jpg.webp)
Oxycodone is available in a variety of formulations for by mouth or under the tongue:[6][25][26][27]
- Immediate-release oxycodone (OxyFast, OxyIR, OxyNorm, Roxicodone)
- Controlled-release oxycodone (OxyContin, Xtampza ER) – 10-12 hour duration[11]
- Oxycodone tamper-resistant (OxyContin OTR)[28]
- Immediate-release oxycodone with paracetamol (acetaminophen) (Percocet, Endocet, Roxicet, Tylox)
- Immediate-release oxycodone with aspirin (Endodan, Oxycodan, Percodan, Roxiprin)
- Immediate-release oxycodone with ibuprofen (Combunox)[29]
- Controlled-release oxycodone with naloxone (Targin, Targiniq, Targinact)[30] – 10-12 hour duration[11]
- Controlled-release oxycodone with naltrexone (Troxyca) – 10-12 hour duration[11][31]
In the US, oxycodone is only approved for use by mouth, available as tablets and oral solutions. Parenteral formulations of oxycodone (brand name OxyNorm) are also available in other parts of the world however, and are widely used in the European Union.[32][33][34] In Spain, the Netherlands and the United Kingdom, oxycodone is approved for intravenous (IV) and intramuscular (IM) use. When first introduced in Germany during World War I, both IV and IM administrations of oxycodone were commonly used for postoperative pain management of Central Powers soldiers.[4]
Side effects
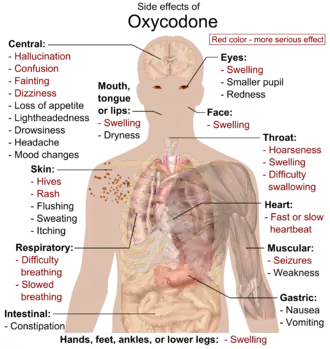

Serious side effects of oxycodone include reduced sensitivity to pain (beyond the pain the drug is taken to reduce), euphoria, anxiolysis, feelings of relaxation, and respiratory depression.[36] Common side effects of oxycodone include constipation (23%), nausea (23%), vomiting (12%), somnolence (23%), dizziness (13%), itching (13%), dry mouth (6%), and sweating (5%).[36][37] Less common side effects (experienced by less than 5% of patients) include loss of appetite, nervousness, abdominal pain, diarrhea, urine retention, dyspnea, and hiccups.[38] Most side effects generally become less intense over time, although issues related to constipation are likely to continue for the duration of use.[39] Oxycodone in combination with naloxone in managed-release tablets, has been formulated to both deter abuse and reduce "opioid-induced constipation".[40]
Dependence and withdrawal
The risk of experiencing severe withdrawal symptoms is high if a patient has become physically dependent and discontinues oxycodone abruptly. Medically, when the drug has been taken regularly over an extended period, it is withdrawn gradually rather than abruptly. People who regularly use oxycodone recreationally or at higher than prescribed doses are at even higher risk of severe withdrawal symptoms. The symptoms of oxycodone withdrawal, as with other opioids, may include "anxiety, panic attack, nausea, insomnia, muscle pain, muscle weakness, fevers, and other flu-like symptoms".[41][42]
Withdrawal symptoms have also been reported in newborns whose mothers had been either injecting or orally taking oxycodone during pregnancy.[43]
Hormone levels
As with other opioids, chronic use of oxycodone (particularly with higher doses) often causes concurrent hypogonadism (low sex hormone levels).[44]
Overdose
In high doses, overdoses, or in some persons not tolerant to opioids, oxycodone can cause shallow breathing, slowed heart rate, cold/clammy skin, pauses in breathing, low blood pressure, constricted pupils, circulatory collapse, respiratory arrest, and death.[38]
In 2011, it was the leading cause of drug-related deaths in the U.S.[45] However, from 2012 onwards, heroin and fentanyl have become more common causes of drug-related deaths.[45]
Oxycodone overdose has also been described to cause spinal cord infarction in high doses and ischemic damage to the brain, due to prolonged hypoxia from suppressed breathing.[46]
Interactions
Oxycodone is metabolized by the enzymes CYP3A4 and CYP2D6, and its clearance therefore can be altered by inhibitors and inducers of these enzymes, increasing and decreasing half-life, respectively.[33] (For lists of CYP3A4 and CYP2D6 inhibitors and inducers, see here and here, respectively.) Natural genetic variation in these enzymes can also influence the clearance of oxycodone, which may be related to the wide inter-individual variability in its half-life and potency.[33]
Ritonavir or lopinavir/ritonavir greatly increase plasma concentrations of oxycodone in healthy human volunteers due to inhibition of CYP3A4 and CYP2D6.[47] Rifampicin greatly reduces plasma concentrations of oxycodone due to strong induction of CYP3A4.[48] There is also a case report of fosphenytoin, a CYP3A4 inducer, dramatically reducing the analgesic effects of oxycodone in a chronic pain patient.[49] Dosage or medication adjustments may be necessary in each case.[47][48][49]
Pharmacology
Pharmacodynamics
| Compound | Affinities (Ki) | Ratio | Ref. | ||
|---|---|---|---|---|---|
| MOR | DOR | KOR | MOR:DOR:KOR | ||
| Oxycodone | 18 nM | 958 nM | 677 nM | 1:53:38 | [4] |
| Oxymorphone | 0.78 nM | 50 nM | 137 nM | 1:64:176 | [50] |
| Compound | Route | Dose |
|---|---|---|
| Codeine | PO | 200 mg |
| Hydrocodone | PO | 20–30 mg |
| Hydromorphone | PO | 7.5 mg |
| Hydromorphone | IV | 1.5 mg |
| Morphine | PO | 30 mg |
| Morphine | IV | 10 mg |
| Oxycodone | PO | 20 mg |
| Oxycodone | IV | 10 mg |
| Oxymorphone | PO | 10 mg |
| Oxymorphone | IV | 1 mg |
Oxycodone, a semi-synthetic opioid, is a highly selective full agonist of the μ-opioid receptor (MOR).[32][33] This is the main biological target of the endogenous opioid neuropeptide β-endorphin.[15] Oxycodone has low affinity for the δ-opioid receptor (DOR) and the κ-opioid receptor (KOR), where it is an agonist similarly.[32][33] After oxycodone binds to the MOR, a G protein-complex is released, which inhibits the release of neurotransmitters by the cell by decreasing the amount of cAMP produced, closing calcium channels, and opening potassium channels.[54] Opioids like oxycodone are thought to produce their analgesic effects via activation of the MOR in the midbrain periaqueductal gray (PAG) and rostral ventromedial medulla (RVM).[55] Conversely, they are thought to produce reward and addiction via activation of the MOR in the mesolimbic reward pathway, including in the ventral tegmental area, nucleus accumbens, and ventral pallidum.[56][57] Tolerance to the analgesic and rewarding effects of opioids is complex and occurs due to receptor-level tolerance (e.g., MOR downregulation), cellular-level tolerance (e.g., cAMP upregulation), and system-level tolerance (e.g., neural adaptation due to induction of ΔFosB expression).[58]
Taken orally, 20 mg of immediate-release oxycodone is considered to be equivalent in analgesic effect to 30 mg of morphine,[59][60] while extended release oxycodone is considered to be twice as potent as oral morphine.[61]
Similarly to most other opioids, oxycodone increases prolactin secretion, but its influence on testosterone levels is unknown.[32] Unlike morphine, oxycodone lacks immunosuppressive activity (measured by natural killer cell activity and interleukin 2 production in vitro); the clinical relevance of this has not been clarified.[32]
Active metabolites
A few of the metabolites of oxycodone have also been found to be active as MOR agonists, some of which notably have much higher affinity for (as well as higher efficacy at) the MOR in comparison.[62][63][64] Oxymorphone possesses 3- to 5-fold higher affinity for the MOR than does oxycodone,[7] while noroxycodone and noroxymorphone possess one-third of and 3-fold higher affinity for the MOR, respectively,[7][64] and MOR activation is 5- to 10-fold less with noroxycodone but 2-fold higher with noroxymorphone relative to oxycodone.[65] Noroxycodone, noroxymorphone, and oxymorphone also have longer biological half-lives than oxycodone.[62][66]
| Compound | Ki | EC50 | Cmax | AUC |
|---|---|---|---|---|
| Oxycodone | 16.0 nM | 343 nM | 23.2 ± 8.6 ng/mL | 236 ± 102 ng/h/mL |
| Oxymorphone | 0.36 nM | 42.8 nM | 0.82 ± 0.85 ng/mL | 12.3 ± 12 ng/h/mL |
| Noroxycodone | 57.1 nM | 1930 nM | 15.2 ± 4.5 ng/mL | 233 ± 102 ng/h/mL |
| Noroxymorphone | 5.69 nM | 167 nM | ND | ND |
| Ki is for [3H]diprenorphine displacement. (Note that diprenorphine is a non-selective opioid receptor ligand, so this is not MOR-specific.) EC50 is for hMOR1 GTPyS binding. Cmax and AUC levels are for 20 mg CR oxycodone. | ||||
However, despite the greater in vitro activity of some of its metabolites, it has been determined that oxycodone itself is responsible for 83.0% and 94.8% of its analgesic effect following oral and intravenous administration, respectively.[63] Oxymorphone plays only a minor role, being responsible for 15.8% and 4.5% of the analgesic effect of oxycodone after oral and intravenous administration, respectively.[63] Although the CYP2D6 genotype and the route of administration result in differential rates of oxymorphone formation, the unchanged parent compound remains the major contributor to the overall analgesic effect of oxycodone.[63] In contrast to oxycodone and oxymorphone, noroxycodone and noroxymorphone, while also potent MOR agonists, poorly cross the blood–brain barrier into the central nervous system, and for this reason are only minimally analgesic in comparison.[62][65][63][64]
κ-opioid receptor
In 1997, a group of Australian researchers proposed (based on a study in rats) that oxycodone acts on KORs, unlike morphine, which acts upon MORs.[67] Further research by this group indicated the drug appears to be a high-affinity κ2b-opioid receptor agonist.[68] However, this conclusion has been disputed, primarily on the basis that oxycodone produces effects that are typical of MOR agonists.[69] In 2006, research by a Japanese group suggested the effect of oxycodone is mediated by different receptors in different situations.[70] Specifically in diabetic mice, the KOR appears to be involved in the antinociceptive effects of oxycodone, while in nondiabetic mice, the μ1-opioid receptor seems to be primarily responsible for these effects.[70][71]
Instant-release absorption profile
Oxycodone can be administered orally, intranasally, via intravenous, intramuscular, or subcutaneous injection, or rectally. The bioavailability of oral administration of oxycodone averages within a range of 60 to 87%, with rectal administration yielding the same results; intranasal varies between individuals with a mean of 46%.[72]
After a dose of conventional (instant-release) oral oxycodone, the onset of action is 10 to 30 minutes,[7][6] and peak plasma levels of the drug are attained within roughly 30 to 60 minutes;[7][6][62] in contrast, after a dose of OxyContin (an oral controlled-release formulation), peak plasma levels of oxycodone occur in about three hours.[38] The duration of instant-release oxycodone is 3 to 6 hours, although this can be variable depending on the individual.[7]
Distribution
Oxycodone has a volume of distribution of 2.6L/kg,[73] in the blood it is distributed to skeletal muscle, liver, intestinal tract, lungs, spleen, and brain.[38] Conventional oral preparations start to reduce pain within 10 to 15 minutes on an empty stomach; in contrast, OxyContin starts to reduce pain within one hour.[12]
Metabolism
The metabolism of oxycodone in humans occurs in the liver mainly via the cytochrome P450 system and is extensive (about 95%) and complex, with many minor pathways and resulting metabolites.[7][74] Around 10% (range 8–14%) of a dose of oxycodone is excreted essentially unchanged (unconjugated or conjugated) in the urine.[7] The major metabolites of oxycodone are noroxycodone (70%), noroxymorphone ("relatively high concentrations"),[36] and oxymorphone (5%).[62][65] The immediate metabolism of oxycodone in humans is as follows:[7][10][75]
- N-Demethylation to noroxycodone predominantly via CYP3A4
- O-Demethylation to oxymorphone predominantly via CYP2D6
- 6-Ketoreduction to 6α- and 6β-oxycodol
- N-Oxidation to oxycodone-N-oxide
In humans, N-demethylation of oxycodone to noroxycodone by CYP3A4 is the major metabolic pathway, accounting for 45% ± 21% of a dose of oxycodone, while O-demethylation of oxycodone into oxymorphone by CYP2D6 and 6-ketoreduction of oxycodone into 6-oxycodols represent relatively minor metabolic pathways, accounting for 11% ± 6% and 8% ± 6% of a dose of oxycodone, respectively.[7][32]
Several of the immediate metabolites of oxycodone are subsequently conjugated with glucuronic acid and excreted in the urine.[7] 6α-Oxycodol and 6β-oxycodol are further metabolized by N-demethylation to nor-6α-oxycodol and nor-6β-oxycodol, respectively, and by N-oxidation to 6α-oxycodol-N-oxide and 6β-oxycodol-N-oxide (which can subsequently be glucuronidated as well).[7][10] Oxymorphone is also further metabolized, as follows:[7][10][75]
- 3-Glucuronidation to oxymorphone-3-glucuronide predominantly via UGT2B7
- 6-Ketoreduction to 6α-oxymorphol and 6β-oxymorphol
- N-Demethylation to noroxymorphone
The first pathway of the above three accounts for 40% of the metabolism of oxymorphone, making oxymorphone-3-glucuronide the main metabolite of oxymorphone, while the latter two pathways account for less than 10% of the metabolism of oxymorphone.[75] After N-demethylation of oxymorphone, noroxymorphone is further glucuronidated to noroxymorphone-3-glucuronide.[75]
Because oxycodone is metabolized by the cytochrome P450 system in the liver, its pharmacokinetics can be influenced by genetic polymorphisms and drug interactions concerning this system, as well as by liver function.[38] Some people are fast metabolizers of oxycodone, resulting in reduced effects, while others are slow metabolizers, resulting in increased effects and toxicity.[76][77] While higher CYP2D6 activity increases the effects of oxycodone (owing to increased conversion into oxymorphone), higher CYP3A4 activity has the opposite effect and decreases the effects of oxycodone (owing to increased metabolism into noroxycodone and noroxymorphone).[78] The dose of oxycodone must be reduced in patients with reduced liver function.[79]
Elimination
The clearance of oxycodone is 0.8 L/min.[80] Oxycodone and its metabolites are mainly excreted in urine.[81] Therefore, oxycodone accumulates in patients with kidney impairment.[79] Oxycodone is eliminated in the urine 10% as unchanged oxycodone, 45% ± 21% as N-demethylated metabolites (noroxycodone, noroxymorphone, noroxycodols), 11 ± 6% as O-demethylated metabolites (oxymorphone, oxymorphols), and 8% ± 6% as 6-keto-reduced metabolites (oxycodols).[81][62]
Duration of action
Oxycodone has a half-life of 4.5 hours.[73] It is available as a generic medication.[12] Its manufacturer Purdue Pharma claimed in their 1992 patent application that the duration of action of OxyContin, oxycodone's controlled-release preparation, is 12 hours in "90% of patients." It has never performed any clinical studies in which OxyContin was given at more frequent intervals. In a separate filing, Purdue claims that controlled-release oxycodone "provides pain relief in said patient for at least 12 hours after administration."[82] However, in 2016 an investigation by the Los Angeles Times found that "the drug weans off hours early in many people," inducing symptoms of opiate withdrawal and intense cravings for OxyContin. One doctor Lawrence Robbins told journalists that over 70% of his patients would report that OxyContin would only provide 4–7 hours of relief. Doctors in the 1990s often would switch their patients to a dosing schedule of once every eight hours when they complained that the duration of action for OxyContin was too short to be only taken twice a day.[82][83]
Purdue strongly discouraged the practice: Purdue's medical director Robert Reder wrote to one doctor in 1995 that "OxyContin has been developed for [12-hour] dosing...I request that you not use a [8-hourly] dosing regimen." Purdue repeatedly released memos to its sales representatives ordering them to remind doctors not to deviate from a 12-hour dosing schedule. One such memo read, "There is no Q8 dosing with OxyContin... [8-hour dosing] needs to be nipped in the bud. NOW!!"[82] The journalists who covered the investigation argue that Purdue Pharma has insisted on a 12-hour duration of action for nearly all patients, despite evidence to the contrary, in order to protect the reputation of OxyContin as a 12-hour drug and the willingness of health insurance and managed care companies to cover OxyContin despite its high cost relative to generic opiates such as morphine.[82]
Purdue sales representatives were instructed to encourage doctors to write prescriptions for larger 12-hour doses instead of more frequent dosing. An August 1996 memo to Purdue sales representatives in Tennessee entitled "$$$$$$$ It’s Bonus Time in the Neighborhood!" reminded the representatives that their commissions would dramatically increase if they were successful in convincing doctors to prescribe larger doses. Los Angeles Times journalists argue using interviews from opioid addiction experts that such high doses of OxyContin spaced 12 hours apart create a combination of agony during opiate withdrawal (lower lows) and a schedule of reinforcement that relieves this agony, fostering addiction.[82]
Chemistry
Oxycodone's chemical name is derived from codeine. The chemical structures are very similar, differing only in that
- Oxycodone has a hydroxy group at carbon-14 (codeine has just a hydrogen in its place)
- Oxycodone has a 7,8-dihydro feature. Codeine has a double bond between those two carbons; and
- Oxycodone has a carbonyl group (as in ketones) in place of the hydroxyl group of codeine.
It is also similar to hydrocodone, differing only in that it has a hydroxyl group at carbon-14.[79]
Oxycodone is marketed as various salts, most commonly as the hydrochloride salt. The free base conversion ratios of different salts are: hydrochloride (0.896), bitartrate (0.667), tartrate (0.750), camphosulfonate (0.576), pectinate (0.588), phenylpriopionate (0.678), sulfate (0.887), phosphate (0.763), and terephthalate (0.792). The hydrochloride salt is the basis of most American oxycodone products whilst bitartrate, tartrate, pectinate, terephthalate and phosphate salts are also available in European Union products. Methyiodide and hydroiodide are mentioned in older European Union publications.
Biosynthesis
In terms of biosynthesis, oxycodone has been found naturally in nectar extracts from the orchid family Epipactis helleborine; together along with another opioid: 3-{2-{3-{3-benzyloxypropyl}-3-indol, 7,8-didehydro- 4,5-epoxy-3,6-d-morphinan.[84]
Detection in biological fluids
Oxycodone and/or its major metabolites may be measured in blood or urine to monitor for clearance, abuse, confirm a diagnosis of poisoning, or assist in a medicolegal death investigation. Many commercial opiate screening tests cross-react appreciably with oxycodone and its metabolites, but chromatographic techniques can easily distinguish oxycodone from other opiates.[85]
History
Martin Freund and (Jakob) Edmund Speyer of the University of Frankfurt in Germany published the first synthesis of oxycodone from thebaine in 1916.[86][17] When Freund died, in 1920, Speyer wrote his obituary.[87] Speyer, born to a Jewish family in Frankfurt am Main in 1878, became a victim of the Holocaust. He died on 5 May 1942, the second day of deportations from the Lodz Ghetto; his death was noted in the ghetto's chronicle.[88]
The first clinical use of the drug was documented in 1917, the year after it was first developed.[17][11] It was first introduced to the U.S. market in May 1939. In early 1928, Merck introduced a combination product containing scopolamine, oxycodone, and ephedrine under the German initials for the ingredients SEE, which was later renamed Scophedal (SCOpolamine, ePHEDrine and eukodAL) in 1942. It was last manufactured in 1987, but can be compounded. This combination is essentially an oxycodone analogue of the morphine-based "twilight sleep", with ephedrine added to reduce circulatory and respiratory effects.[89] The drug became known as the "Miracle Drug of the 1930s" in Continental Europe and elsewhere and it was the Wehrmacht's choice for a battlefield analgesic for a time. The drug was expressly designed to provide what the patent application and package insert referred to as "very deep analgesia and profound and intense euphoria" as well as tranquillisation and anterograde amnesia useful for surgery and battlefield wounding cases. Oxycodone was allegedly chosen over morphine, hydromorphone, and hydrocodone for this product because of oxycodone having subjective elements in its side effect profile similar to cocaine.[90]
During Operation Himmler, Skophedal was also reportedly injected in massive overdose into the prisoners dressed in Polish Army uniforms in the staged incident on 1 September 1939 which opened the Second World War.[89][91]
The personal notes of Adolf Hitler's physician, Theodor Morell, indicate Hitler received repeated injections of "eukodal" (oxycodone) and Scophedal, as well as Dolantin (pethidine) codeine, and morphine less frequently; oxycodone could not be obtained after late January 1945.[92][93]
In the early 1970s, the U.S. government classified oxycodone as a schedule II drug.
Purdue Pharma — a privately held company based in Stamford, Connecticut, developed the prescription painkiller OxyContin. Upon its release in 1995, OxyContin was hailed as a medical breakthrough, a long-lasting narcotic that could help patients suffering from moderate to severe pain. The drug became a blockbuster, and has reportedly generated some US$35 billion in revenue for Purdue.[94]
Society and culture
Lawsuits
In October 2017, The New Yorker published a story on Mortimer Sackler and Purdue Pharma regarding their ties to the production and manipulation of the oxycodone markets.[94] The article links Raymond and Arthur Sackler's business practices with the rise of direct pharmaceutical marketing and eventually to the rise of addiction to oxycodone in the United States. The article implies that Sackler bears some responsibility for the opioid epidemic in the United States.[95] In 2019 the New York Times ran a piece confirming Sackler told company officials in 2008 to “measure our performance by Rx’s by strength, giving higher measures to higher strengths.”[96] This was verified with documents tied to a lawsuit – which was filed by the Massachusetts attorney general, Maura Healey – claiming that Purdue Pharma and members of the Sackler family knew that high doses of OxyContin over long periods would increase the risk of serious side effects, including addiction.[97] Despite Purdue Pharma's proposal for a US$12 billion settlement of the lawsuit, the attorneys general of 23 states, including Massachusetts, rejected the settlement offer in September, 2019.[98]
Legal status
General
Oxycodone is subject to international conventions on narcotic drugs. In addition, oxycodone is subject to national laws that differ by country. The 1931 Convention for Limiting the Manufacture and Regulating the Distribution of Narcotic Drugs of the League of Nations included oxycodone.[99] The 1961 Single Convention on Narcotic Drugs of the United Nations, which replaced the 1931 convention, categorized oxycodone in Schedule I.[100] Global restrictions on Schedule I drugs include "limit[ing] exclusively to medical and scientific purposes the production, manufacture, export, import, distribution of, trade in, use and possession of" these drugs; "requir[ing] medical prescriptions for the supply or dispensation of [these] drugs to individuals"; and "prevent[ing] the accumulation" of quantities of these drugs "in excess of those required for the normal conduct of business".[100]
Australia
Oxycodone is in Schedule I (derived from the Single Convention on Narcotic Drugs) of the Commonwealth's Narcotic Drugs Act 1967.[101] In addition, it is in Schedule 8 of the Australian Standard for the Uniform Scheduling of Drugs and Poisons ("Poisons Standard"), meaning it is a "controlled drug... which should be available for use but require[s] restriction of manufacture, supply, distribution, possession and use to reduce abuse, misuse and physical or psychological dependence".[102]
Canada
Oxycodone is a controlled substance under Schedule I of the Controlled Drugs and Substances Act (CDSA).[103]
Canadian legislative changes
In February 2012, Ontario passed legislation to allow the expansion of an already existing drug-tracking system for publicly funded drugs to include those that are privately insured. This database will function to identify and monitor patient's attempts to seek prescriptions from multiple doctors or retrieve from multiple pharmacies. Other provinces have proposed similar legislation, while some, such as Nova Scotia, have legislation already in effect for monitoring prescription drug use. These changes have coincided with other changes in Ontario's legislation to target the misuse of painkillers and high addiction rates to drugs such as oxycodone. As of February 29, 2012, Ontario passed legislation delisting oxycodone from the province's public drug benefit program. This was a first for any province to delist a drug based on addictive properties. The new law prohibits prescriptions for OxyNeo except to certain patients under the Exceptional Access Program including palliative care and in other extenuating circumstances. Patients already prescribed oxycodone will receive coverage for an additional year for OxyNeo, and after that, it will be disallowed unless designated under the exceptional access program.[104]
Much of the legislative activity has stemmed from Purdue Pharma's decision in 2011 to begin a modification of Oxycontin's composition to make it more difficult to crush for snorting or injecting. The new formulation, OxyNeo, is intended to be preventive in this regard and retain its effectiveness as a painkiller. Since introducing its Narcotics Safety and Awareness Act, Ontario has committed to focusing on drug addiction, particularly in the monitoring and identification of problem opioid prescriptions, as well as the education of patients, doctors, and pharmacists.[105] This Act, introduced in 2010, commits to the establishment of a unified database to fulfil this intention.[106] Both the public and medical community have received the legislation positively, though concerns about the ramifications of legal changes have been expressed. Because laws are largely provincially regulated, many speculate a national strategy is needed to prevent smuggling across provincial borders from jurisdictions with looser restrictions.[107]
In 2015, Purdue Pharma's abuse-resistant OxyNEO and six generic versions of OxyContin had been on the Canada-wide approved list for prescriptions since 2012. In June 2015, then federal Minister of Health Rona Ambrose announced that within three years all oxycodone products sold in Canada would need to be tamper-resistant. Some experts warned that the generic product manufacturers may not have the technology to achieve that goal, possibly giving Purdue Pharma a monopoly on this opiate.[108]
Canadian lawsuits
Several class action suits across Canada have been launched against the Purdue group of companies and affiliates. Claimants argue the pharmaceutical manufacturers did not meet a standard of care and were negligent in doing so. These lawsuits reference earlier judgments in the United States, which held that Purdue was liable for wrongful marketing practices and misbranding. Since 2007, the Purdue companies have paid over CAN$650 million in settling litigation or facing criminal fines.
Germany
The drug is in Appendix III of the Narcotics Act (Betäubungsmittelgesetz or BtMG).[109] The law allows only physicians, dentists, and veterinarians to prescribe oxycodone and the federal government to regulate the prescriptions (e.g., by requiring reporting).[109]
Hong Kong
Oxycodone is regulated under Part I of Schedule 1 of Hong Kong's Chapter 134 Dangerous Drugs Ordinance.[110]
Japan
Oxycodone is a restricted drug in Japan. Its import and export is strictly restricted to specially designated organizations having prior permit to import it. In a high-profile case an American who was a top Toyota executive living in Tokyo, who claimed to be unaware of the law, was arrested for importing oxycodone into Japan.[111][112]
Singapore
Oxycodone is listed as a Class A drug in the Misuse of Drugs Act of Singapore, which means offences in relation to the drug attract the most severe level of punishment. A conviction for unauthorized manufacture of the drug attracts a minimum sentence of 10 years of imprisonment and corporal punishment of 5 strokes of the cane, and a maximum sentence of life imprisonment or 30 years of imprisonment and 15 strokes of the cane.[113] The minimum and maximum penalties for unauthorized trafficking in the drug are respectively 5 years of imprisonment and 5 strokes of the cane, and 20 years of imprisonment and 15 strokes of the cane.[114]
United Kingdom
Oxycodone is a Class A drug under the Misuse of Drugs Act.[115] For Class A drugs, which are "considered to be the most likely to cause harm", possession without a prescription is punishable by up to seven years in prison, an unlimited fine, or both.[116] Dealing of the drug illegally is punishable by up to life imprisonment, an unlimited fine, or both.[116] In addition, oxycodone is a Schedule 2 drug per the Misuse of Drugs Regulations 2001 which "provide certain exemptions from the provisions of the Misuse of Drugs Act 1971".[117]
United States
Under the Controlled Substances Act, oxycodone is a Schedule II controlled substance whether by itself or part of a multi-ingredient medication.[118] The DEA lists oxycodone both for sale and for use in manufacturing other opioids as ACSCN 9143 and in 2013 approved the following annual aggregate manufacturing quotas: 131.5 metric tons for sale, down from 153.75 in 2012, and 10.25 metric tons for conversion, unchanged from the previous year.[119] In 2020, oxycodone possession was decriminalized in the U.S. state of Oregon.[120]
Effects
Oxycodone, like other opioid analgesics, tends to induce feelings of euphoria, relaxation and reduced anxiety in those who are occasional users.[121] These effects make it one of the most commonly abused pharmaceutical drugs in the United States.[122]
Preventive measures
In August 2010, Purdue Pharma reformulated their long-acting oxycodone line, marketed as OxyContin, using a polymer, Intac,[123] to make the pills extremely difficult to crush or dissolve[124] in water to reduce OxyContin abuse.[125] The FDA approved relabeling the reformulated version as abuse-resistant in April 2013.[126]
Pfizer manufactures a preparation of short-acting oxycodone, marketed as Oxecta, which contains inactive ingredients, referred to as tamper-resistant Aversion Technology.[127] It does not deter oral abuse. Approved by the FDA in the U.S. in June 2011, the new formulation makes crushing, chewing, snorting, or injecting the opioid impractical because of a change in its chemical properties.[128]
Australia
The non-medical use of oxycodone existed from the early 1970s, but by 2015, 91% of a national sample of injecting drug users in Australia had reported using oxycodone, and 27% had injected it in the last six months.[129]
Canada
Opioid-related deaths in Ontario had increased by 242% from 1969 to 2014.[130] By 2009 in Ontario there were more deaths from oxycodone overdose than from cocaine overdose.[131] Deaths from opioid pain relievers had increased from 13.7 deaths per million residents in 1991 to 27.2 deaths per million residents in 2004.[132] The abuse of oxycodone in Canada became a problem. Areas where oxycodone is most problematic are Atlantic Canada and Ontario, where its abuse is prevalent in rural towns, and in many smaller to medium-sized cities.[133] Oxycodone is also widely available across Western Canada, but methamphetamine and heroin are more serious problems in the larger cities, while oxycodone is more common in rural towns. Oxycodone is diverted through doctor shopping, prescription forgery, pharmacy theft, and overprescribing.[133][134]
The recent formulations of oxycodone, particularly Purdue Pharma's crush-, chew-, injection- and dissolve-resistant OxyNEO[135] which replaced the banned OxyContin product in Canada in early 2012, have led to a decline in the abuse of this opiate but have increased the abuse of the more potent drug fentanyl.[136] According to a Canadian Centre on Substance Abuse study quoted in Maclean's magazine, there were at least 655 fentanyl-related deaths in Canada in a five-year period.[137]
In Alberta, the Blood Tribe police claimed that from the fall of 2014 through January 2015, oxycodone pills or a lethal fake variation referred to as Oxy 80s[138] containing fentanyl made in illegal labs by members of organized crime were responsible for ten deaths on the Blood Reserve, which is located southwest of Lethbridge, Alberta.[139] Province-wide, approximately 120 Albertans died from fentanyl-related overdoses in 2014.[138]
United Kingdom
Abuse and diversion of oxycodone in the UK commenced in the early- to mid-2000s.[140] The first known death due to overdose in the UK occurred in 2002.[141] However, recreational use remains relatively rare.
United States
In the United States, more than 12 million people use opioid drugs recreationally.[142] Opioids were responsible for 49,000 of the 72,000 drug overdose deaths in the U.S. in 2017.[143] In September 2013, the FDA released new labeling guidelines for long acting and extended release opioids requiring manufacturers to remove moderate pain as indication for use, instead stating the drug is for "pain severe enough to require daily, around-the-clock, long term opioid treatment."[144] The updated labeling will not restrict physicians from prescribing opioids for moderate, as needed use.[142]
Oxycodone is the most widely recreationally used opioid in America. The U.S. Department of Health and Human Services estimates that about 11 million people in the U.S. consume oxycodone in a non-medical way annually.[145] In 2007, about 42,800 emergency room visits occurred due to "episodes" involving oxycodone.[146] Diverted oxycodone may be taken orally or ingested through insufflation; used intravenously, or the heated vapors inhaled. In 2008, recreational use of oxycodone and hydrocodone were involved in 14,800 deaths. Some of the cases were due to overdoses of the acetaminophen component, resulting in fatal liver damage.[147]
Reformulated OxyContin is causing some recreational users to change to heroin, which is cheaper and easier to obtain.[148]
Economics
The International Narcotics Control Board estimated 11.5 short tons (10.4 t) of oxycodone were manufactured worldwide in 1998;[149] by 2007 this figure had grown to 75.2 short tons (68.2 t).[149] United States accounted for 82% of consumption in 2007 at 51.6 short tons (46.8 t). Canada, Germany, Australia, and France combined accounted for 13% of consumption in 2007.[149][150] In 2010, 1.3 short tons (1.2 t) of oxycodone were illegally manufactured using a fake pill imprint. This accounted for 0.8% of consumption. These illicit tablets were later seized by the U.S. Drug Enforcement Administration, according to the International Narcotics Control Board.[151] The board also reported 122.5 short tons (111.1 t) manufactured in 2010. This number had decreased from a record high of 135.9 short tons (123.3 t) in 2009.[152]
Names
Expanded expressions for the compound oxycodone in the academic literature include "dihydrohydroxycodeinone",[1][153][154] "Eucodal",[153][154] "Eukodal",[4][11] "14-hydroxydihydrocodeinone",[1][153] and "Nucodan".[153][154] In a UNESCO convention, the translations of "oxycodone" are oxycodon (Dutch), oxycodone (French), oxicodona (Spanish), الأوكسيكودون (Arabic), 羟考酮 (Chinese), and оксикодон (Russian).[155] The word "oxycodone" should not be confused with "oxandrolone", "oxazepam", "oxybutynin", "oxytocin", or "Roxanol".[156]
Other brand names include Longtec and Shortec.[157]
References
- O'Neil MJ, ed. (2006). The Merck index (14th ed.). Whitehouse Station, NJ: Merck & Co. ISBN 978-0-911910-00-1.
- "Oxycodone Use During Pregnancy". Drugs.com. 14 October 2019. Retrieved 12 April 2020.
- Bonewit-West K, Hunt SA, Applegate E (2012). Today's Medical Assistant: Clinical and Administrative Procedures. Elsevier Health Sciences. p. 571. ISBN 9781455701506.
- Kalso E (May 2005). "Oxycodone". Journal of Pain and Symptom Management. 29 (5 Suppl): S47-56. doi:10.1016/j.jpainsymman.2005.01.010. PMID 15907646.
- "Roxicodone, OxyContin (oxycodone) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Retrieved 8 April 2014.
- Elliott JA, Smith HS (19 April 2016). Handbook of Acute Pain Management. CRC Press. pp. 82–. ISBN 978-1-4665-9635-1.
- Smith H, Passik S (25 April 2008). Pain and Chemical Dependency. Oxford University Press USA. pp. 195–. ISBN 978-0-19-530055-0.
- "Roxicodone, OxyContin (oxycodone) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Retrieved 4 January 2019.
- Yarbro CH, Wujcik D, Gobel BH (15 November 2010). Cancer Nursing: Principles and Practice. Jones & Bartlett Publishers. pp. 695–. ISBN 978-1-4496-1829-2.
- McPherson RA, Pincus MR (31 March 2016). Henry's Clinical Diagnosis and Management by Laboratory Methods. Elsevier Health Sciences. pp. 336–. ISBN 978-0-323-41315-2.
- Sunshine A, Olson NZ, Colon A, Rivera J, Kaiko RF, Fitzmartin RD, et al. (July 1996). "Analgesic efficacy of controlled-release oxycodone in postoperative pain". Journal of Clinical Pharmacology. 36 (7): 595–603. doi:10.1002/j.1552-4604.1996.tb04223.x. PMID 8844441. S2CID 35076787.
Treatment with CR oxycodone was safe and effective in this study, and its characteristics will be beneficial in the treatment of pain.
- "Oxycodone Monograph for Professionals". Drugs.com. AHFS. Retrieved 28 December 2018.
- Pergolizzi JV, Taylor R, LeQuang JA, Raffa RB (2018). "Managing severe pain and abuse potential: the potential impact of a new abuse-deterrent formulation oxycodone/naltrexone extended-release product". Journal of Pain Research. 11: 301–311. doi:10.2147/JPR.S127602. PMC 5810535. PMID 29445297.
- British national formulary : BNF 74 (74 ed.). British Medical Association. 2017. p. 442. ISBN 978-0857112989.
- Talley NJ, Frankum B, Currow D (10 February 2015). Essentials of Internal Medicine 3e. Elsevier Health Sciences. pp. 491–. ISBN 978-0-7295-8081-6.
- "Stanford School of Medicine, Palliative Care, Opioid Conversion / Equivalency Table". 2013-04-20. Cite journal requires
|journal=(help) - Sneader, W. (2005). Drug discovery: a history. Hoboken, NJ: Wiley. p. 119. ISBN 978-0-471-89980-8.
- "The Top 300 of 2020". ClinCalc. Retrieved 11 April 2020.
- "Oxycodone - Drug Usage Statistics". ClinCalc. Retrieved 11 April 2020.
- Dart RC, Iwanicki JL, Dasgupta N, Cicero TJ, Schnoll SH (2017). "Do abuse deterrent opioid formulations work?". Journal of Opioid Management. 13 (6): 365–378. doi:10.5055/jom.2017.0415. PMID 29308584.
- Riley J, Eisenberg E, Müller-Schwefe G, Drewes AM, Arendt-Nielsen L (January 2008). "Oxycodone: a review of its use in the management of pain". Current Medical Research and Opinion. 24 (1): 175–92. doi:10.1185/030079908X253708. PMID 18039433. S2CID 9099037.
- Biancofiore G (September 2006). "Oxycodone controlled release in cancer pain management" (PDF). Therapeutics and Clinical Risk Management. 2 (3): 229–34. doi:10.2147/tcrm.2006.2.3.229. PMC 1936259. PMID 18360598. Retrieved January 30, 2019.
- Hanks GW, Conno F, Cherny N, Hanna M, Kalso E, McQuay HJ, et al. (March 2001). "Morphine and alternative opioids in cancer pain: the EAPC recommendations". British Journal of Cancer. 84 (5): 587–93. doi:10.1054/bjoc.2001.1680. PMC 2363790. PMID 11237376.
- "FDA approves OxyContin for kids 11 to 16". www.msn.com. Archived from the original on 30 June 2017. Retrieved 11 February 2018.
- Gould III HJ (11 December 2006). Understanding Pain: What It Is, Why It Happens, and How It's Managed. Demos Medical Publishing. pp. 71–. ISBN 978-1-934559-82-6.
- Graves K (29 September 2015). Drug I.D. & Symptom Guide 6th Edition QWIK-CODE. LawTech Publishing Group. pp. 99–. ISBN 978-1-56325-225-9.
- Linda Skidmore-Roth (16 July 2015). Mosby's Drug Guide for Nursing Students, with 2016 Update. Elsevier Health Sciences. pp. 789–. ISBN 978-0-323-17297-4.
- "accessdata.fda.gov" (PDF). Retrieved 11 February 2018.
- Sinatra RS, de Leon-Cassasola OA (27 April 2009). Acute Pain Management. Cambridge University Press. pp. 198–. ISBN 978-0-521-87491-5.
- Staats PS, Silverman SM (28 May 2016). Controlled Substance Management in Chronic Pain: A Balanced Approach. Springer. pp. 172–. ISBN 978-3-319-30964-4.
- "FDA Approves Troxyca® ER (Oxycodone Hydrochloride and Naltrexone Hydrochloride) Extended-release Capsules CII with Abuse-deterrent Properties for the Management of Pain". 19 August 2016.
- Mellar P. Davis (28 May 2009). Opioids in Cancer Pain. OUP Oxford. pp. 155–158. ISBN 978-0-19-923664-0.
- Karen Forbes (29 November 2007). Opioids in Cancer Pain. OUP Oxford. pp. 64–65. ISBN 978-0-19-921880-6.
- Bradbury H, Hodge BS (8 November 2013). Practical Prescribing for Medical Students. SAGE Publications. pp. 93–. ISBN 978-1-4462-9753-7.
- American Society of Health-System Pharmacists (2009-03-23). "Oxycodone". U.S. National Library of Medicine, MedlinePlus. Retrieved 2009-03-27.
- Fitzgibbon DR, Loeser JD (28 March 2012). Cancer Pain. Lippincott Williams & Wilkins. pp. 198–. ISBN 978-1-4511-5279-1.
- "Oxycodone Side Effects". Drugs.com. Retrieved 22 May 2013.
- 1. Package insert Oxycontin (PDF). Stamford, CT: Purdue Pharma L.P. 2007-11-05. Archived from the original (PDF) on 2009-03-26. Retrieved 2009-03-23.
- Ordóñez Gallego A, González Barón M, Espinosa Arranz E (May 2007). "Oxycodone: a pharmacological and clinical review". Clinical & Translational Oncology. 9 (5): 298–307. doi:10.1007/s12094-007-0057-9. PMID 17525040.
- Simpson K, Leyendecker P, Hopp M, Müller-Lissner S, Löwenstein O, De Andrés J, et al. (December 2008). "Fixed-ratio combination oxycodone/naloxone compared with oxycodone alone for the relief of opioid-induced constipation in moderate-to-severe noncancer pain". Current Medical Research and Opinion. 24 (12): 3503–12. doi:10.1185/03007990802584454. PMID 19032132. S2CID 73061000.
- "Oxycodone". Center for Substance Abuse Research. 2005-05-02. Retrieved 2009-03-25.
- "Tapering off opioids: When and how". Mayo Clinic. Retrieved 10 October 2020.
- Rao R, Desai NS (June 2002). "OxyContin and neonatal abstinence syndrome". Journal of Perinatology. 22 (4): 324–5. doi:10.1038/sj.jp.7210744. PMID 12032797.
- Brennan MJ (March 2013). "The effect of opioid therapy on endocrine function". The American Journal of Medicine. 126 (3 Suppl 1): S12-8. doi:10.1016/j.amjmed.2012.12.001. PMID 23414717.
- "Drugs Most Frequently Involved in Drug Overdose Deaths: United States, 2011–2016" (PDF). CDC. 12 December 2018. Retrieved 21 December 2018.
- Ntranos A, Shoirah H, Dhamoon MS, Hahn D, Naidich TP, Shin S (March 2017). "Clinical Reasoning: A young woman with respiratory failure, hearing loss, and paraplegia". Neurology. 88 (10): e78–e84. doi:10.1212/WNL.0000000000003684. PMID 28265044. Retrieved 2017-03-09.
- Nieminen TH, Hagelberg NM, Saari TI, Neuvonen M, Neuvonen PJ, Laine K, Olkkola KT (October 2010). "Oxycodone concentrations are greatly increased by the concomitant use of ritonavir or lopinavir/ritonavir". European Journal of Clinical Pharmacology. 66 (10): 977–85. doi:10.1007/s00228-010-0879-1. PMID 20697700. S2CID 25770818.
- Nieminen TH, Hagelberg NM, Saari TI, Pertovaara A, Neuvonen M, Laine K, et al. (June 2009). "Rifampin greatly reduces the plasma concentrations of intravenous and oral oxycodone". Anesthesiology. 110 (6): 1371–8. doi:10.1097/ALN.0b013e31819faa54. PMID 19417618.
- Pon D, Hwang J, Lo T, Zyl CV (2015). "Decreased responsiveness to oxycodone: A case of a pharmacokinetic drug interaction?". Journal of Opioid Management. 11 (4): 357–61. doi:10.5055/jom.2015.0284. PMID 26312962.
- Corbett AD, Paterson SJ, Kosterlitz HW (1993). Opioids. Handbook of Experimental Pharmacology. 104. pp. 645–679. doi:10.1007/978-3-642-77460-7_26. ISBN 978-3-642-77462-1. ISSN 0171-2004.
- King (25 October 2010). Pharmacology for Women's Health. Jones & Bartlett Publishers. pp. 332–. ISBN 978-1-4496-1073-9.
- Chestnut DH, Wong CA, Tsen LC, Ngan Kee WD, Beilin Y, Mhyre J (28 February 2014). Chestnut's Obstetric Anesthesia: Principles and Practice E-Book. Elsevier Health Sciences. pp. 611–. ISBN 978-0-323-11374-8.
- Adriana P. Tiziani (1 June 2013). Havard's Nursing Guide to Drugs. Elsevier Health Sciences. pp. 933–. ISBN 978-0-7295-8162-2.
- Chahl L (1996). "Opioids- mechanism of action". Aust Prescr. 19 (3): 63–65. doi:10.18773/austprescr.1996.063.
- Christoph Stein (1999). Opioids in Pain Control: Basic and Clinical Aspects. Cambridge University Press. pp. 46–. ISBN 978-0-521-62269-1.
- Squire L, Berg D, Bloom FE, du Lac S, Ghosh A, Spitzer NC (17 December 2012). Fundamental Neuroscience. Academic Press. pp. 884–. ISBN 978-0-12-385871-9.
- Kringelbach ML, Berridge KC (2010). Pleasures of the Brain. Oxford University Press. pp. 33–. ISBN 978-0-19-533102-8.
- Sinatra RS, Jahr JS, Watkins-Pitchford JM (14 October 2010). The Essence of Analgesia and Analgesics. Cambridge University Press. pp. 167–. ISBN 978-1-139-49198-3.
- "Treatment of Pain". Merck Manuals Professional Edition. Retrieved 2016-04-24.
- Ferrell BR, Pasero C, McCaffery M (2010). "Table 16-1 Equianalgesic Dose Chart". Pain Assessment and Pharmacologic Management. Elsevier Health Sciences. ISBN 978-0323082631.
- Levy EF, Victor J (2007). Opioids in medicine a comprehensive review on the mode of action and the use of analgesics in different clinical pain states. New York: Springer Science+Business Media B.V. p. 371. ISBN 978-1402059476.
- Lalovic B, Kharasch E, Hoffer C, Risler L, Liu-Chen LY, Shen DD (May 2006). "Pharmacokinetics and pharmacodynamics of oral oxycodone in healthy human subjects: role of circulating active metabolites". Clinical Pharmacology and Therapeutics. 79 (5): 461–79. doi:10.1016/j.clpt.2006.01.009. PMID 16678548. S2CID 21372271.
- Klimas R, Witticke D, El Fallah S, Mikus G (May 2013). "Contribution of oxycodone and its metabolites to the overall analgesic effect after oxycodone administration". Expert Opinion on Drug Metabolism & Toxicology. 9 (5): 517–28. doi:10.1517/17425255.2013.779669. PMID 23488585. S2CID 22857902.
- Lemberg KK, Siiskonen AO, Kontinen VK, Yli-Kauhaluoma JT, Kalso EA (February 2008). "Pharmacological characterization of noroxymorphone as a new opioid for spinal analgesia". Anesthesia and Analgesia. 106 (2): 463–70, table of contents. doi:10.1213/ane.0b013e3181605a15. PMID 18227301. S2CID 16524280.
- Victor R. Preedy (25 April 2016). Neuropathology of Drug Addictions and Substance Misuse Volume 3: General Processes and Mechanisms, Prescription Medications, Caffeine and Areca, Polydrug Misuse, Emerging Addictions and Non-Drug Addictions. Elsevier Science. pp. 462–464. ISBN 978-0-12-800677-1.
- Firestein GS, Budd RC, Gabriel SE, McInnes IB, O'Dell JR (21 June 2016). Kelley and Firestein's Textbook of Rheumatology. Elsevier Health Sciences. pp. 1080–. ISBN 978-0-323-31696-5. LCCN 2016009254.
- Ross FB, Smith MT (November 1997). "The intrinsic antinociceptive effects of oxycodone appear to be kappa-opioid receptor mediated". Pain. 73 (2): 151–7. doi:10.1016/S0304-3959(97)00093-6. PMID 9415500. S2CID 53165907.
- Smith MT (October 2008). "Differences between and combinations of opioids re-visited". Current Opinion in Anesthesiology. 21 (5): 596–601. doi:10.1097/ACO.0b013e32830a4c4a. PMID 18784485. S2CID 14293344.
- Kalso E (December 2007). "How different is oxycodone from morphine?". Pain. 132 (3): 227–8. doi:10.1016/j.pain.2007.09.027. PMID 17961923. S2CID 45689872.
- Nozaki C, Saitoh A, Kamei J (March 2006). "Characterization of the antinociceptive effects of oxycodone in diabetic mice". European Journal of Pharmacology. 535 (1–3): 145–51. doi:10.1016/j.ejphar.2006.02.002. PMID 16533506.
- Nozaki C, Kamei J (April 2007). "Involvement of mu1-opioid receptor on oxycodone-induced antinociception in diabetic mice". European Journal of Pharmacology. 560 (2–3): 160–2. doi:10.1016/j.ejphar.2007.01.021. PMID 17292346.
- Analgesic Expert Group. Therapeutic Guidelines: Analgesic. Version 4. Melbourne: Therapeutic Guidelines Ltd, 2007.
- "Oxycodone". www.drugbank.ca. Retrieved 24 January 2019.
- Moore KA, Ramcharitar V, Levine B, Fowler D (September 2003). "Tentative identification of novel oxycodone metabolites in human urine". Journal of Analytical Toxicology. 27 (6): 346–52. doi:10.1093/jat/27.6.346. PMID 14516487.
- Anzenbacher P, Zanger UM (29 May 2012). Metabolism of Drugs and Other Xenobiotics. John Wiley & Sons. pp. 420–. ISBN 978-3-527-32903-8.
- Gasche Y, Daali Y, Fathi M, Chiappe A, Cottini S, Dayer P, Desmeules J (December 2004). "Codeine intoxication associated with ultrarapid CYP2D6 metabolism". The New England Journal of Medicine. 351 (27): 2827–31. doi:10.1056/NEJMoa041888. PMID 15625333.
- Otton SV, Wu D, Joffe RT, Cheung SW, Sellers EM (April 1993). "Inhibition by fluoxetine of cytochrome P450 2D6 activity". Clinical Pharmacology and Therapeutics. 53 (4): 401–9. doi:10.1038/clpt.1993.43. PMID 8477556. S2CID 39724277.
- Samer CF, Daali Y, Wagner M, Hopfgartner G, Eap CB, Rebsamen MC, et al. (June 2010). "Genetic polymorphisms and drug interactions modulating CYP2D6 and CYP3A activities have a major effect on oxycodone analgesic efficacy and safety". British Journal of Pharmacology. 160 (4): 919–30. doi:10.1111/j.1476-5381.2010.00709.x. PMC 2935998. PMID 20590588.
- "Oxycodone". The American Society of Health-System Pharmacists. Retrieved 3 April 2011.
- "Oxycodone". www.drugbank.ca. Retrieved 24 January 2019.
- Davis PJ, Cladis FP (15 October 2016). Smith's Anesthesia for Infants and Children E-Book. Elsevier Health Sciences. pp. 234–. ISBN 978-0-323-38869-6.
- Ryan H, Girion L, Glover S (7 July 2016). "You want a description of hell?' OxyContin's 12-hour problem". Los Angeles Times. Retrieved 8 July 2018.
- "'Q12' Workshops, 2001". Los Angeles Times. Retrieved 8 July 2018.
- "Why do pollinators become 'sluggish'? Nectar chemical constituents from Epipactis helleborine L. Crantz Orchidaceae". Applied Ecology & Environmental Research. 2005;3(2):29-38. Jakubska A, Przado D, Steininger M, Aniol-Kwiatkowska A, Kadej M.
- Baselt R (2017). Disposition of Toxic Drugs and Chemicals in Man (11th ed.). Foster City, CA: Biomedical Publications. pp. 1604–1607.
- Freund M, Speyer E (24 November 1916). "Über die Umwandlung von Thebain in Oxycodeinon und dessen Derivate". Journal für Praktische Chemie. 94 (1): 135–178. doi:10.1002/prac.19160940112.
- "Martin Freund". Berichte der Deutschen Chemischen Gesellschaft (A and B Series). 54 (5): A53–A79. 7 May 1921. doi:10.1002/cber.19210540533.
- The Holocaust : a history of the Jews of Europe during the Second World War (1st American ed.). Holt, Rinehart, and Winston. 1986. pp. 346. ISBN 0-03-062416-9. Retrieved 3 June 2020.
- Defalque RJ, Wright AJ (October 2003). "Scophedal (SEE) was it a fad or a miracle drug?". Bulletin of Anesthesia History. 21 (4): 12–4. doi:10.1016/S1522-8649(03)50051-8. PMID 17494237.
- William S Burroughs 1952 letter to Allen Ginsburg concerning Eukodal, in Collected Correspondance, pp 141-2
- Merck 1930 package insert for Skophedal (German)
- Breitenbach, Dagmar (9 September 2015). "A fresh light on the Nazis' wartime drug addiction". Deutsche Welle. Retrieved 2016-04-24.
- Ohler N, Whiteside S (2017). Blitzed: drugs in the Third Reich (First U.S. ed.). Boston: Houghton Mifflin Harcourt. p. 194. ISBN 978-1-328-66379-5.
- Keefe PR (2017-10-23). "The Family That Built an Empire of Pain". The New Yorker. ISSN 0028-792X. Retrieved 2019-02-02.
- Keefe PR (23 October 2017). "The Family That Built an Empire of Pain". The New Yorker. ISSN 0028-792X. Retrieved 18 November 2017.
- Meier B (2019-01-31). "Sackler Scion's Email Reveals Push for High-Dose OxyContin, New Lawsuit Disclosures Claim". The New York Times. ISSN 0362-4331. Retrieved 2019-02-03.
- Bebinger M, Willmsen C (15 January 2019). "Mass. AG Implicates Family Behind Purdue Pharma In Opioid Deaths". WBUR Boston. Retrieved 19 September 2019.
- DeCosta-Klipa N (17 September 2019). "Maura Healey explains why she refused to join the Purdue Pharma settlement". Boston.com. Boston Globe Media Partners, LLC. Retrieved 19 September 2019.
- League of Nations (1931). "Convention for limiting the manufacture and regulating the distribution of narcotic drugs" (PDF). Retrieved 2009-04-04.
- "United Nations conference for the adoption of a single convention on narcotic drugs. Final act" (PDF). 1961. Archived from the original (PDF) on 2011-08-17. Retrieved 2009-04-04.
- Commonwealth of Australia. "Narcotic Drugs Act 1967 – first schedule". Australasian Legal Information Institute. Retrieved 2009-04-06.
- Australian Government. Department of Health and Aging. Therapeutic Goods Administration (June 2008). Standard for the uniform scheduling of drugs and poisons no. 23 (PDF). Canberra: Commonwealth of Australia. ISBN 978-1-74186-596-7. Retrieved 2009-04-06.
- Canada Department of Justice (2009-02-27). "Controlled Drugs and Substances Act (1996, c. 19)". Retrieved 2009-03-23.
- Olgilvie, Megan. "Ontario delisting OxyContin and its substitute from drug benefit program" Toronto Star (2012-02-17)
- Narcotics Safety and Awareness Act. 2010. Ministry of Health and Long Term Care.
- Dhalla I, Born K (2012-02-22). "Opioids". healthydebate.ca. Archived from the original on March 21, 2015.
- Ontario OxyContin Rules: New Restrictions Applauded But National Rules Needed. Huffington Post. Canadian Press (2012-02-20)
- Weeks C, Howlett K (August 4, 2015). "New oxycodone rules would give drug maker a monopoly in Canada, experts warn". Globe and Mail. Toronto, Ontario. Retrieved December 15, 2015.
- German Federal Ministry of Justice (2009-01-19). "Act on the circulation of narcotics (Narcotics Act – BtMG)" (in German). Retrieved 2009-04-06.
- Hong Kong Special Administrative Region, People's Republic of China. "Dangerous drugs ordinance – chapter 134". Hong Kong Legal Information Institute. Archived from the original on 2007-12-15. Retrieved 2009-04-08.
- "Toyota's American PR chief arrested for suspected drug violation". Retrieved 11 February 2018.
- "Toyota: American exec did not intend to break Japan law". Archived from the original on 19 June 2015. Retrieved 11 February 2018.
- Misuse of Drugs Act (Cap. 185, 2008 Rev. Ed.) (Singapore), section 6(1).
- Misuse of Drugs Act (Singapore), section 5(1).
- "List of drugs currently controlled under the Misuse of Drugs legislation" (PDF). UK. Home Office. 2009. Archived from the original (PDF) on 2007-02-05. Retrieved 2009-04-08.
- "Class A, B and C drugs". UK. Home Office. Archived from the original on 2007-08-04. Retrieved 2009-04-08.
- "Statutory instrument 2001 No. 3998. The Misuse of Drugs regulations 2001". UK. Office of Public Sector Information. Retrieved 2009-04-08.
- DEA. "Controlled substance scheduling". Drug information and scheduling. Drug Enforcerment Administration. Archived from the original on 21 November 2015. Retrieved 23 November 2015.
- "DEA Diversion Control CSA". US Dept of Justice – DEA. Retrieved 23 May 2013.
- Andrew Selsky (4 November 2020). "Oregon leads the way in decriminalizing hard drugs". The Associated Press. Retrieved 7 November 2020.
- "OxyContin: Pain Relief vs. Abuse". Retrieved 11 February 2018.
- "Top 10 Most Commonly Abused Prescription Medications". 28 April 2014. Retrieved 11 February 2018.
- "New Abuse Deterrent Formulation Technology for Immediate-Release Opiods" (PDF). Grünenthal Group. Grünenthal Group Worldwide. 2010. Archived from the original (PDF) on 2015-12-22. Retrieved December 15, 2015.
- Diep F (May 13, 2013). "How Do You Make a Painkiller Addiction-Proof". Popular Science. Bonnier Corporation. Retrieved January 30, 2019.
- Coplan P (2012). Findings from Purdue's Post-Marketing Epidemiology Studies of Reformulated OxyContin's Effects (PDF). NASCSA 2012 Conference. Scottsdale, Arizona. Archived from the original (PDF) on June 14, 2013.
- "Press Announcements; FDA approves abuse-deterrent labeling for reformulated OxyContin". US Government – FDA. Retrieved 23 May 2013.
- "Pfizer and Acura Announce FDA Approval of Oxectatm (Oxycodone HCL, USP) CII". Pfizer News and Media. Pfizer Inc. Archived from the original on December 22, 2015. Retrieved December 15, 2015.
- Fiore K (June 20, 2011). "FDA Okays New Abuse-Resistant Opioid". MedPage Today. MedPage Today. Archived from the original on December 22, 2015. Retrieved December 15, 2015.
- Black E (2008). Australian drug trends 2007. Findings from the Illicit Drug Reporting System (IDRS) (PDF). Sydney: National Drug and Alcohol Research Centre, University of New South Wales. ISBN 978-0-7334-2625-4. Archived from the original (PDF) on 2008-07-21.
- Boyle T (7 July 2014), "Opioid deaths soaring, study finds Opioid-related deaths in Ontario jumped by a whopping 242 per cent over two decades, according to a study by ICES and St. Mike's", The Star, Toronto, Ontario, retrieved 23 January 2015
- Donovan K (10 February 2009), "Oxycodone found to be more deadly than heroin", The Star, Toronto, Ontario, retrieved 23 January 2015
- "Study finds huge rise in oxycodone deaths". CTV News. Retrieved 2009-12-07.
- "OxyContin Fact Sheet" (PDF). ccsa.ca. Archived from the original (PDF) on 2008-11-17. Retrieved 2012-05-10.
- "Health Canada – Misuse and Abuse of Oxycodone-based Prescription Drugs". Hc-sc.gc.ca. 2010-01-11. Archived from the original on 2011-11-26. Retrieved 2012-05-10.
- Kirkey, Sharon (May 23, 2012). "OxyNEO another prescription for disaster?". Globe and Mail. Toronto, Ontario.
- Criger, Erin (August 17, 2015). "Death of OxyContin behind rise of fentanyl?". CityNews. Rogers Digital Media. Retrieved February 7, 2019.
- Gatehouse J, Macdonald N (June 22, 2015). "Fentanyl: The King of all Opiates, and a Killer Drug Crisis". Macleans. Rogers Media. Retrieved December 15, 2015.
- Southwick R (December 2, 2015). "Fentanyl brings tragedy to Blood Tribe". Calgary Herald. Calgary, Alberta. Archived from the original on December 22, 2015. Retrieved December 15, 2015.
- Police believe organized crime is flooding the Blood Tribe reserve with an illegal drug that has been linked to 10 deaths, Alberta, 23 January 2015, archived from the original on 2016-01-24, retrieved 23 January 2015
- Gordon T (2008-03-30). "Scots' use of 'hillbilly heroin' rises by 430%". Sunday Times (London).
- Thompson T (2002-03-24). "Epidemic fear as 'hillbilly heroin' hits the streets". Society Guardian. Retrieved 2009-04-16.
- Girioin L, Haely, Melissa (11 September 2013). "FDA to require stricter labeling for pain drugs". Los Angeles Times. pp. A1 and A9.
- "Overdose Death Rates". www.drugabuse.gov. 29 January 2019. Retrieved 11 May 2019.
- "ER/LA Opioid Analgesic Class Labeling Changes and Postmarket Requirements" (PDF). FDA. Retrieved 12 September 2013.
- Now a counselor, she went from stoned to straight, San Francisco Chronicle, November 2. 2015.
- "Oxycontin and Addiction". consumer.healthday.com. Retrieved 2016-04-24.
- Policy Impact: Prescription Pain Killer Overdoses Centers for Disease Control and Prevention. Retrieved 24 December 2013.
- Reformulated OxyContin reduces abuse but many addicts have switched to heroin, The Pharmaceutical Journal, 16 March 2015.
- International Narcotics Control Board (2009). Narcotic drugs: estimated world requirements for 2009; statistics for 2007. Report E/INCB/2008/2 (PDF). New York: United Nations. ISBN 978-92-1-048124-3.
- "Availability of Opioid Analgesics in the World and Asia, With a special focus on: Indonesia, Philippines, Thailand" (PDF). University of Wisconsin Pain & Policy Studies Group/World Health Organization (WHO) Collaborating Center for Policy and Communications in Cancer Care. United Nations. Archived from the original (PDF) on 26 April 2012. Retrieved 27 November 2011.
- Narcotic Drugs: Estimated World Requirements for 2012 and Statistics for 2010. International Narcotics Control Board (2011).
- Narcotic Drugs: Estimated World Requirements for 2012 and Statistics for 2010. International Narcotics Control Board (2011).
- Eddy NB (1973). The National Research Council involvement in the opiate problem, 1928–1971. Washington: National Academy of Sciences.
- May EL, Jacobson AE (June 1989). "The Committee on Problems of Drug Dependence: a legacy of the National Academy of Sciences. A historical account". Drug and Alcohol Dependence. 23 (3): 183–218. doi:10.1016/0376-8716(89)90083-5. PMID 2666074.
- United Nations Educational, Scientific; Cultural Organization (2005). "International convention against doping in sport" (PDF). Retrieved 2009-04-04.
- Hicks RW, Becker SC, Cousins DD, eds. (2008). MEDMARX data report. A report on the relationship of drug names and medication errors in response to the Institute of Medicine's call for action (PDF). Rockville, MD: Center for the Advancement of Patient Safety, US Pharmacopeia. Retrieved 2009-04-04.
- Postlethwaite J. "Oxycodone Longtec Patient Leaflet" (PDF). Great Western Hospitals NHS Foundation Trust. Great Western Hospitals NHS Foundation Trust. Retrieved 23 March 2019.
Further reading
- Coluzzi F, Mattia C (July–August 2005). "Oxycodone. Pharmacological profile and clinical data in chronic pain management" (PDF). Minerva Anestesiologica. 71 (7–8): 451–60. PMID 16012419. Archived from the original (PDF) on March 9, 2006.
External links
- "Oxycodone". Drug Information Portal. U.S. National Library of Medicine.
- "Oxycodone hydrochloride". Drug Information Portal. U.S. National Library of Medicine.