Oxygen-evolving complex
The oxygen-evolving complex (OEC), also known as the water-splitting complex, is a water-oxidizing enzyme involved in the photo-oxidation of water during the light reactions of photosynthesis.[2] OEC is surrounded by 4 core proteins of photosystem II at the membrane-lumen interface.[3] Electrons extracted from 2 water molecules along with 4 protons produce a singular O2 molecule.[3] The molecular machinery for dissecting water requires the ability to store energy from the first three photons before the fourth provides sufficient energy for water oxidation.[4] Then, the OEC transfers 4 electrons, 1 at a time, to photosystem II via a tyrosine residue in the reaction center.[4] Based on a widely accepted theory from 1970 by Bessel Kok, the complex can exist in 5 states: S0 to S4. S4 being the most oxidized and S0 the most reduced. Photons trapped by photosystem II move the system from state S0 to S4. S4 is unstable and reacts with water producing free oxygen. For the complex to reset to the lowest state, S0, it uses 2 water molecules to pull out 4 electrons.[3]

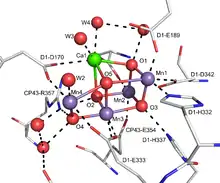
The OEC is composed of three protein subunits, OEE1 (PsbO), OEE2 (PsbP) and OEE3 (PsbQ); a fourth PsbR peptide is associated nearby. The OEC appears to have a metalloenzyme core containing both manganese and calcium, with the empirical formula for the inorganic core of Mn4Ca1OxCl1–2(HCO3)y. This cluster is coordinated by D1 and CP43 subunits and stabilized by peripheral membrane proteins.[3] Other characteristics of it have been reviewed; see [5]
Currently, the mechanism of the complex is not completely understood.[6] The exact order in which manganese oxidation occurs through the photo-induced Kok cycle is unknown.[3] Along with the role of Ca+2, Cl−1, and the membrane proteins surrounding the metal cluster not being well understood. Much of what is known has been collected from flash experiments, EPR, and x-ray spectroscopy.[7]
It is suggested that the evolution of the OEC was triggered by the presence of the manganese-containing minerals rancieite and hollandite in the early oceans. The minerals were, it is presumed, assimilated by the early cyanobacteria, which have incorporated them in the active center of the complex.
In order to determine more about the structure, most research focuses on creating manganese-core dimers at various protonation states and comparing the resultant data with observed behavior of the complex.[8]
Location
The OEC is only found in chloroplasts and cyanobacteria. Within the chloroplasts, the OEC is inserted in the thylakoid membrane, composed of both granal thylakoids and stromal thylakoids.[4]
References
- Umena, Yasufumi; Kawakami, Keisuke; Shen, Jian-Ren; Kamiya, Nobuo (May 2011). "Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9 Å" (PDF). Nature. 473 (7345): 55–60. doi:10.1038/nature09913. PMID 21499260.
- Raymond, J.; Blankenship, R. (2008). "The origin of the oxygen-evolving complex". Coordination Chemistry Reviews. 252 (3–4): 377–383. doi:10.1016/j.ccr.2007.08.026.
- Amin, Muhamed. "Computational Insights Into The Oxygen Evolving Complex Of Photosystem II".
- Johnson, James. "The Origin of Life - The Rise of the Oxygen Evolving Complex". www.chm.bris.ac.uk. Florida University. Retrieved 2020-04-30.
- Abstract : Manganese: The Oxygen-Evolving Complex & Models1 : Encyclopedia of Inorganic Chemistry : Wiley InterScience
- Yano, Junko; Kern, Jan; Yachandra, Vittal K.; Nilsson, Håkan; Koroidov, Sergey; Messinger, Johannes (2015). "Chapter 2, Section 2 Geometric and Electronic Structure of the Mn4CaO5 Cluster". In Peter M.H. Kroneck and Martha E. Sosa Torres (ed.). Sustaining Life on Planet Earth: Metalloenzymes Mastering Dioxygen and Other Chewy Gases. Metal Ions in Life Sciences. 15. Springer. pp. 13–43. doi:10.1007/978-3-319-12415-5_2. ISBN 978-3-319-12414-8. PMC 4688042. PMID 25707465.
- Kok, B.; Forbush, B.; McGloin, M. (1970). "Cooperation of charges in photosynthetic O2 evolution. I. A linear four-step mechanism". Photochem. Photobiol. 11 (6): 467–475. doi:10.1111/j.1751-1097.1970.tb06017.x. PMID 5456273.
- Cooper, Daniel Steven (2009). Towards models for the oxygen evolving centre of photosystem II and analysis of Lewis acidic metals within novel salen derivatives (phd thesis). Cardiff University.
Further reading
- 'Life Ascending' by Nick Lane, Profile Books 2009; pp 83–87 - outlines a possible route for the evolution of the OEC.