Peficitinib
Peficitinib (Smyraf) is a pharmaceutical drug used for the treatment of rheumatoid arthritis. It belongs to the class of drugs known as Janus kinase inhibitors (JAK inhibitors).[1][2]
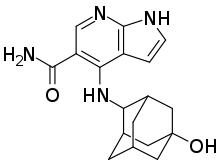 | |
| Clinical data | |
|---|---|
| Trade names | Smyraf |
| Other names | ASP015K; JNJ-54781532 |
| Legal status | |
| Legal status |
|
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
| Formula | C18H22N4O2 |
| Molar mass | 326.400 g·mol−1 |
Peficitinib was approved for use in Japan in 2019.[3]
References
- Kivitz AJ, Gutierrez-Ureña SR, Poiley J, Genovese MC, Kristy R, Shay K, et al. (April 2017). "Peficitinib, a JAK Inhibitor, in the Treatment of Moderate-to-Severe Rheumatoid Arthritis in Patients With an Inadequate Response to Methotrexate". Arthritis & Rheumatology. 69 (4): 709–719. doi:10.1002/art.39955. PMID 27748083.
- Genovese MC, Greenwald M, Codding C, Zubrzycka-Sienkiewicz A, Kivitz AJ, Wang A, et al. (May 2017). "Peficitinib, a JAK Inhibitor, in Combination With Limited Conventional Synthetic Disease-Modifying Antirheumatic Drugs in the Treatment of Moderate-to-Severe Rheumatoid Arthritis". Arthritis & Rheumatology. 69 (5): 932–942. doi:10.1002/art.40054. PMID 28118538.
- Markham A, Keam SJ (June 2019). "Peficitinib: First Global Approval". Drugs. 79 (8): 887–891. doi:10.1007/s40265-019-01131-y. PMID 31093950.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.