Persicarin
Persicarin is a sulfated flavonoid naturally found in the cells of several plant species, including the water dropwort (Oenanthe javanica). It was first isolated from water pepper in 1937 by Jeffrey Harborne.[1] The name of persicarin is derived from the Latin name of the plant: Persicaria hydropiper. It is also found in dill and because it is then excreted into urine, that makes persicarin a potential biomarker for the consumption of this food.[2] Persicarin has been reported to show some promising results in protecting against and treating type 1 diabetes-induced liver inflammation and damage in mice models.[3]
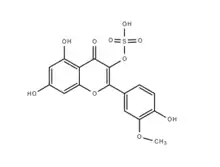 Skeletal structure of persicarin | |
| Names | |
|---|---|
| IUPAC name
5,7-Dihydroxy-2-(4-hydroxy-3-methoxyphenyl)-4-oxo-4H-chromen-3-yl hydrogen sulfate | |
| Other names
isohamnetin 3-monosulphate Isohamnetin 3-sulfate Isohamnetin 3-monosulfate Chromone, 2-(4-hydroxy-3-methoxyphenyl)-3,5,7-trihydroxy-, 3-sulfate (ester) 4H-1-Benzopyran-4-one, 5,7-dihydroxy-2-(4-hydroxy-3-methoxyphenyl)-3-(sulfooxy)- | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C16H12O10S | |
| Molar mass | 396.32 g·mol−1 |
| Density | 1.9±0.1 g/cm3 |
| Melting point | 288 °C (550 °F; 561 K) decomposes at 288 at 280mmHg 101 °C (214 °F; 374 K)) |
| practically insoluble in water | |
| Acidity (pKa) | -3.53 |
Refractive index (nD) |
1.764 |
| Hazards | |
| Main hazards | Toxic |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Persicarin is poorly soluble but is potentially a very strongly acidic substance based on its estimated pKa value.
The functional role played by persicarin in plant cells and tissues, and in fact the role of other sulfated flavonoids in general, is currently not clear. It has been theorised that sulfated flavonoids play a role in molecular recognition, stimulate plant hormone transport, detoxification and signalling pathways.[4][5][6] Sulfated flavonoids seem to also have an important role in co-pigmentation by forming stable complexes with anthocyanin pigments, but the specific function of persicarin remains unknown.[7]
References
- Harborne, Jeffrey B. (June 1975). "Flavonoid sulphates: A new class of sulphur compounds in higher plants". Phytochemistry. 14 (5–6): 1147–1155. doi:10.1016/S0031-9422(00)98585-6.
- "Showing Compound Persicarin (FDB016887) - FooDB". foodb.ca. Retrieved 2020-02-21.
- Lee, Joo Young; Kim, Min Yeong; Shin, Sung Ho; Shin, Mi-Rae; Kwon, O Jun; Kim, Tae Hoon; Park, Chan Hum; Noh, Jeong Sook; Rhee, Man Hee; Roh, Seong-Soo (April 2017). "Persicarin isolated from Oenanthe javanica protects against diabetes-induced oxidative stress and inflammation in the liver of streptozotocin-induced type 1 diabetic mice". Experimental and Therapeutic Medicine. 13 (4): 1194–1202. doi:10.3892/etm.2017.4113. ISSN 1792-0981. PMC 5377288. PMID 28413457.
- Teles, Yanna; Horta, Carolina; Agra, Maria; Siheri, Weam; Boyd, Marie; Igoli, John; Gray, Alexander; de Souza, Maria (2015-11-09). "New Sulphated Flavonoids from Wissadula periplocifolia (L.) C. Presl (Malvaceae)". Molecules. 20 (11): 20161–20172. doi:10.3390/molecules201119685. ISSN 1420-3049. PMC 6332353. PMID 26569200.
- Ananvoranich, S.; Varin, L.; Gulick, P.; Ibrahim, R. (1994-10-01). "Cloning and Regulation of Flavonol 3-Sulfotransferase in Cell-Suspension Cultures of Flaveria bidentis". Plant Physiology. 106 (2): 485–491. doi:10.1104/pp.106.2.485. ISSN 0032-0889. PMC 159553. PMID 7991681.
- Barron, Denis; Varin, Luc; Ibrahim, Ragai K.; Harborne, Jeffrey B.; Williams, Christine A. (January 1988). "Sulphated flavonoids—an update". Phytochemistry. 27 (8): 2375–2395. doi:10.1016/0031-9422(88)87003-1.
- Teles, Yanna C. F.; Souza, Maria Sallett R.; de Souza, Maria de Fátima Vanderlei (2018-02-23). "Sulphated Flavonoids: Biosynthesis, Structures, and Biological Activities". Molecules. 23 (2): 480. doi:10.3390/molecules23020480. ISSN 1420-3049. PMC 6017314. PMID 29473839.