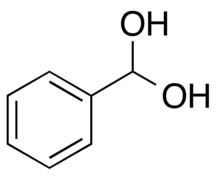
Phenylmethanediol
Phenylmethanediol is an organic compound that is a geminal diol, the hydrate of benzaldehyde. It is a short-lived intermediate in some chemical reactions, such as oxidations of toluene[2] and benzaldehyde[3] and the reduction of benzoic acid.[4]
 | |
| Names | |
|---|---|
| IUPAC name
Phenylmethanediol | |
Other names
| |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C7H8O2 | |
| Molar mass | 124.139 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
References
- "Phenylmethanediol | C7H8O2". ChemSpider.
- Pritchard, Huw O. (2006). "On the atmospheric oxidation of liquid toluene". Physical Chemistry Chemical Physics. 8 (39): 4559–4562.
- Nigam, S. K.; Khan, M. U.; Tiwari, Saras; Dwivedi, H. P.; Singh, P. K. (2004). "Kinetics and mechanism of oxidation of benzaldehyde and substituted benzaldehyde by N-chlorosaccharin". Asian Journal of Chemistry. 16 (2): 761–766.CS1 maint: multiple names: authors list (link)
- Cheng, Po-Chung; Nonaka, Tsutomu (1995). "Kinetic study of the electroreduction of benzoic acid". Bulletin of the Chemical Society of Japan. 68 (1): 378–84.CS1 maint: multiple names: authors list (link)
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.