Pinacolborane
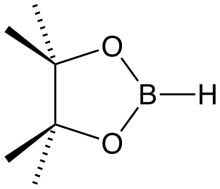
Pinacolborane is the borane with the formula (CH3)4C2O2BH. It features a five-membered C2O2B ring. Owing to the presence of the alkoxide substituents, it is a monomer. It is a colorless liquid prepared by treating borane adducts with pinacol.[1] It features a reactive B-H functional group.[2]
 | |
| Names | |
|---|---|
| IUPAC name
4,4,5,5-Tetramethyl-1,3,2-dioxaborolane | |
| Other names
HBpin | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| EC Number |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C6H13BO2 | |
| Molar mass | 127.98 g·mol−1 |
| Appearance | colorless liquid |
| Density | 0.882 g/cm3 |
| Boiling point | 42–43 °C (108–109 °F; 315–316 K) 50 mmHg |
| Hazards | |
| GHS pictograms |    |
| GHS Signal word | Danger |
| H220, H225, H260, H261, H315, H318 | |
| P210, P223, P231+232, P233, P240, P241, P242, P243, P264, P280, P302+352, P303+361+353, P305+351+338, P310, P321, P332+313, P335+334, P362, P370+378, P377, P381, P402+404, P403, P403+235, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Use in organic synthesis
In the presence of catalysts, pinacolborane hydroborates alkenes and, less rapidly, alkynes.[2][3]
Pinacolborane also effects catalyst-free hydroboration of aldehydes,[4] ketones,[5] and carboxylic acids.[6]
Pinacolborane is used in borylation, a form of C-H activation.[7][8]
Related compounds
References
- Ramachandran, P. Veeraraghavan; Chandra, J. Subash; Ros, Abel; Fernández, Rosario; Lassaleta, José M. (2014). "Pinacolborane". Encyclopedia of Reagents for Organic Synthesis. pp. 1–7. doi:10.1002/047084289X.rn00574.pub2. ISBN 9780470842898.
- Brown, H.C.; Zaidlewicz, M. (2001). Organic Syntheses Via Boranes, Vol. 2. Milwaukee, WI: Aldrich Chemical Co. ISBN 978-0-9708441-0-1.
- Ely, Robert J.; Morken, James P. (2011). "Stereoselective Nickel-Catalyzed 1,4-Hydroboration of 1,3-Dienes". Organic Syntheses. 88: 342. doi:10.15227/orgsyn.088.0342.
- Stachowiak, Hanna; Kaźmierczak, Joanna; Kuciński, Krzysztof; Hreczycho, Grzegorz (2018). "Catalyst-free and solvent-free hydroboration of aldehydes". Green Chemistry. 20 (8): 1738–1742. doi:10.1039/C8GC00042E. ISSN 1463-9262.
- Wang, Weifan; Luo, Man; Yao, Weiwei; Ma, Mengtao; Pullarkat, Sumod A.; Xu, Li; Leung, Pak-Hing (2019). "Catalyst-free and solvent-free hydroboration of ketones". New Journal of Chemistry. 43 (27): 10744–10749. doi:10.1039/C9NJ02722J. ISSN 1144-0546.
- Harinath, Adimulam; Bhattacharjee, Jayeeta; Panda, Tarun K. (2019). "Facile reduction of carboxylic acids to primary alcohols under catalyst-free and solvent-free conditions". Chemical Communications. 55 (10): 1386–1389. doi:10.1039/C8CC08841A. ISSN 1359-7345. PMID 30607398.
- Amaike, K.; Loach, R. P.; Movassaghi, M. (2015). "Direct C7 Functionalization of Tryptophan. Synthesis of Methyl (S)-2-((tert-Butoxycarbonyl)amino)-3-(7-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-indol-3-yl)propanoate". Organic Syntheses. 92: 373–385. doi:10.15227/orgsyn.092.0373 (inactive 2021-01-11). PMC 4733874. PMID 26839440.CS1 maint: DOI inactive as of January 2021 (link)
- Ishiyama, Tatsuo; Takagi, Jun; Nobuta, Yusuke; Miyaura, Norio (2005). "Iridium-Catalyzed C-H Borylation of Arenes and Heteroarenes: 1-Chloro-3-Iodo-5-(4,4,5,5-Tetramethyl-1,3,2-Dioxaborolan-2-Yl)Benzene and 2-(4,4,5,5,-Tetramethyl-1,3,2-Dioxaborolan-2-Yl)Indole". Organic Syntheses. 82: 126. doi:10.15227/orgsyn.082.0126. hdl:2115/56319.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.