Polyestradiol phosphate
Polyestradiol phosphate (PEP), sold under the brand name Estradurin, is an estrogen medication which is used primarily in the treatment of prostate cancer in men.[1][9][2][10] It is also used in women to treat breast cancer, as a component of hormone therapy to treat low estrogen levels and menopausal symptoms, and as a component of feminizing hormone therapy for transgender women.[1][11] It is given by injection into muscle once every four weeks.[1][2][12]
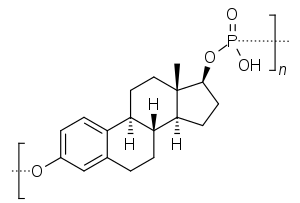 | |
 Skeletal structure of polyestradiol phosphate (top) and ball-and-stick model of estradiol phosphate (one monomer of polyestradiol phosphate) (bottom) | |
| Clinical data | |
|---|---|
| Trade names | Estradurin, Estradurine |
| Other names | PEP; Estradiol phosphate polymer; Estradiol 17β-phosphate polymer; Estradiol polymer with phosphoric acid; Leo-114 |
| AHFS/Drugs.com | International Drug Names |
| Pregnancy category |
|
| Routes of administration | Intramuscular injection[1][2] |
| Drug class | Estrogen; Estrogen ester |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | IM: High |
| Protein binding | Estradiol: ~98% (to albumin and SHBG)[3][4][1] |
| Metabolism | Mainly in the liver, to a lesser extent in the kidneys, gonads, and muscle (by phosphatases)[1] |
| Metabolites | Estradiol, phosphoric acid, and metabolites of estradiol[5][6] |
| Elimination half-life | PEP: 70 days (10 weeks)[7] Estradiol: 1–2 hours[8] |
| Excretion | Urine (as conjugates)[1] |
| Identifiers | |
| |
| CAS Number | |
| PubChem SID | |
| DrugBank | |
| ChemSpider |
|
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | (C18H23O4P)n (n = variable; n = 13) |
| Molar mass | Polymer: Variable Repeat unit: 334.347 g/mol |
| Melting point | 195 to 202 °C (383 to 396 °F) |
| (verify) | |
Common side effects of PEP include headache, breast tenderness, breast development, feminization, sexual dysfunction, infertility, and vaginal bleeding.[1][2] PEP is a synthetic estrogen and hence is an agonist of the estrogen receptor, the biological target of estrogens like estradiol.[2][6][5] It is an estrogen ester in the form of a polymer and is an extremely long-lasting prodrug of estradiol in the body.[2][12][5][6] The time it takes for 50% of a dose of PEP to be eliminated from the body is more than two months.[7] Because PEP works by being converted into estradiol, it is considered to be a natural and bioidentical form of estrogen.[5][2] The safety profile of parenteral estradiol esters like PEP is greatly improved relative to synthetic oral estrogens like ethinylestradiol and diethylstilbestrol.[2]
PEP was discovered around 1953 and was introduced for medical use in the United States in 1957.[12][13][14] Along with estradiol undecylate and estradiol valerate, it has been frequently used in the United States and Europe as a parenteral form of estrogen to treat men with prostate cancer.[15] However, it is no longer available in the United States.[13][16]
Medical uses
PEP is used as an intramuscular injection for estrogen therapy of prostate cancer in men.[1][2] It is also used to treat breast cancer in women who are at least 5 years postmenopausal.[1][17][18] In addition, PEP is used in hormone replacement therapy for low estrogen levels due to hypogonadism or menopause in women.[1] It is also used in feminizing hormone therapy for transgender women.[11][19] PEP is a form of high-dose estrogen therapy.[2] After an injection, it very slowly releases the active agent estradiol over at least several months.[20][7]
PEP has been compared to combined androgen blockade (CAB; castration plus flutamide) for the treatment of prostate cancer in a large randomized clinical trial of 915 patients.[21][22] At 18.5 months, there was no difference in survival or cardiovascular toxicity between the two treatment modalities.[21][22] These findings suggest that parenteral forms of estradiol may have similar effectiveness and safety relative to androgen deprivation therapy (ADT) in the treatment of prostate cancer.[21][22] In addition, estrogens may have significant advantages relative to ADT in terms of bone loss and fractures, hot flashes, sexual function, and quality of life, as well as considerable cost savings with parenteral forms of estradiol compared to GnRH analogue therapy.[21][22] On the other hand, breast tenderness and gynecomastia occur at very high rates with estrogens, whereas incidences are low with castration and CAB.[23] However, gynecomastia with estrogens is generally only mild-to-moderate in severity and is usually only modestly discomforting.[2] In addition, gynecomastia caused by estrogens can be prevented with prophylactic irradiation of the breasts or can be remediated with mastectomy.[2]
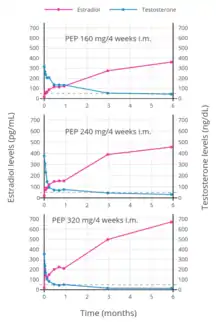
PEP has been studied for the treatment of prostate cancer at dosages of 160 mg/month (three studies) and 240 mg/month (four studies).[24] At a dosage of 160 mg/month, PEP incompletely suppresses testosterone levels, failing to reach the castrate range, and is significantly inferior to orchiectomy in slowing disease progression.[24][2] Conversely, PEP at a dosage of 240 mg/month results in greater testosterone suppression, into the castrate range similarly to orchiectomy, and is equivalent to orchiectomy in effectiveness.[24][2]
For prostate cancer in men, PEP is usually given at a dosage of 80 to 320 mg every 4 weeks for the first 2 to 3 months to rapidly build up estradiol levels.[1] Thereafter, to maintain estradiol levels, the dosage is adjusted down usually to 40 to 160 mg every 4 weeks based on clinical findings and laboratory parameters.[1] For breast cancer and low estrogen levels in women, the dosage is 40 to 80 mg every 4 weeks.[1] For transgender women, the dosage is 80 to 160 mg every 4 weeks.[11][19][25][5]
Available forms
PEP is provided in the form of powder or an aqueous solution in vials and ampoules alone or in combination with mepivacaine and/or nicotinamide (vitamin B3) for administration via intramuscular injection.[1][26][27] Mepivacaine is a local anaesthetic and is used to avoid a burning sensation during injection of PEP.[1] Each vial/ampoule of Estradurin contains 80 mg PEP, 5 mg mepivacaine hydrochloride, 40 mg nicotinamide, and 2 mL water.[27]
Contraindications
The contraindications of PEP are largely the same as those of estradiol and include:[1][28][29][30][31]
- Hypersensitivity to PEP, mepivacaine, or other ingredients
- Known, previous, or suspected breast cancer or other estrogen-dependent malignant tumors (e.g., endometrial cancer)
- Vaginal bleeding of unknown cause or untreated endometrial hyperplasia
- Thrombosis and related, including active thrombophlebitis, former or current venous thromboembolism (deep vein thrombosis, pulmonary embolism), active or recent arterial thromboembolism (e.g., angina, myocardial infarction), or known thrombophilia (e.g., protein C deficiency, protein S deficiency, antithrombin deficiency)
- Severe arrhythmia, hypotension, hypertension, or lipid metabolism disorders
- Cerebrovascular events (i.e., stroke)
- Acute liver disease or previously confirmed liver disease, with abnormal liver function tests or jaundice (e.g., Dubin–Johnson syndrome, Rotor syndrome)
- Severe hepatic dysfunction
- Others including porphyria, sickle cell anemia, otosclerosis, or myasthenia gravis
- Pregnancy, lactation, and breastfeeding
Side effects
Systematic studies of the side effects of PEP are lacking.[1] However, its side effects are assumed to be identical to those of estradiol and other estradiol esters.[1] The side effects of PEP are partially dependent on sex.[1] Common or frequent (>10%) side effects are considered to include headache, abdominal pain, nausea, rash, pruritus, loss of libido, erectile dysfunction, breast tenderness, gynecomastia, feminization, demasculinization, infertility, and vaginal bleeding or spotting.[1][32] Side effects that occur occasionally or uncommonly (0.1–1%) include sodium and water retention, edema, hypersensitivity, breast tension, depression, dizziness, visual disturbances, palpitations, dyspepsia, erythema nodosum, urticaria, and chest pain.[1] All other side effects of PEP are considered to be rare.[1]
The rare (<0.1%) side effects of PEP are considered to include weight gain, impaired glucose tolerance, mood changes (elation or depression), nervousness, tiredness, headache, migraine, intolerance of contact lenses, hypertension, thrombosis, thrombophlebitis, thromboembolism, heart failure, myocardial infarction, vomiting, bloating, cholestatic jaundice, cholelithiasis, transient increases in transaminases and bilirubin, erythema multiforme, hyperpigmentation, muscle cramps, dysmenorrhea, vaginal discharge, premenstrual-like symptoms, breast enlargement, testicular atrophy, allergic reactions (e.g., urticaria, bronchial asthma, anaphylactic shock) due to mepivacaine, and injection site reactions (e.g., pain, sterile abscesses, inflammatory infiltrates).[1]
As thromboembolic and other cardiovascular complications are associated mainly with synthetic oral estrogens like ethinylestradiol and diethylstilbestrol, they occur much less often with parenteral bioidentical forms of estrogen like PEP.[1][2]
Cardiovascular effects
PEP produces minimal undesirable effects on coagulation factors and is thought to increase the risk of blood clots little or not at all.[33][34] This is in spite of the fact that estradiol levels can reach high concentrations of as much as 700 pg/mL with high-dose (320 mg/month) PEP therapy.[35] It is also in contrast to oral synthetic estrogens such as diethylstilbestrol and ethinylestradiol, which produce marked increases in coagulation factors and high rates of blood clots at the high doses used to achieve castrate levels of testosterone in prostate cancer.[33][34][6] The difference between the two types of therapies is due to the bioidentical and parenteral nature of PEP and its minimal influence on liver protein synthesis.[33][34][6] PEP might actually reduce the risk of blood clots, due to decreases in levels of certain procoagulatory proteins.[33][34] Although PEP does not increase the hepatic production or levels of procoagulatory factors, it has been found to significantly decrease levels of the anticoagulatory antithrombin III, which may indicate a potential risk of thromboembolic and cardiovascular complications.[2] On the other hand, PEP significantly increases levels of HDL cholesterol and significantly decreases levels of LDL cholesterol, changes which are thought to protect against coronary artery disease.[2] It appears that PEP may have beneficial effects on cardiovascular health at lower dosages (e.g., 160 mg/day) due to its beneficial effects on HDL and LDL cholesterol levels, but these are overshadowed at higher dosages (e.g., 240 mg/day) due to unfavorable dose-dependent effects on hemostasis, namely antithrombin III levels.[2]
Small early pilot studies of PEP for prostate cancer in men found no cardiovascular toxicity with the therapy.[33] A dosage of PEP of 160 mg/month specifically does not appear to increase the risk of cardiovascular complications.[2] In fact, potential beneficial effects on cardiovascular mortality have been observed at this dosage.[2] However, PEP at a higher dosage of 240 mg/month has subsequently been found in large studies to significantly increase cardiovascular morbidity relative to GnRH modulators and orchiectomy in men treated with it for prostate cancer.[33][34][2] The increase in cardiovascular morbidity with PEP therapy is due to an increase in non-fatal cardiovascular events, including ischemic heart disease and heart decompensation, specifically heart failure.[34][36][37] Conversely, PEP has not been found to significantly increase cardiovascular mortality relative to GnRH modulators and orchiectomy.[33][34] Moreover, numerically more patients with preexisting cardiovascular disease were randomized to the PEP group in one large study (17.1% vs. 14.5%; significance not reported), and this may have contributed to the increased incidence of cardiovascular morbidity observed with PEP.[34] In any case, some studies have found that the increased cardiovascular morbidity with PEP is confined mainly to the first one or two years of therapy, whereas one study found consistently increased cardiovascular morbidity across three years of therapy.[33] A longitudinal risk analysis that projected over 10 years suggested that the cardiovascular risks of PEP may be reversed with long-term treatment and that the therapy may eventually result in significantly decreased cardiovascular risk relative to GnRH modulators and orchiectomy, although this has not been confirmed.[33]
The cardiovascular toxicity of PEP is far less than that of oral synthetic estrogens like diethylstilbestrol and ethinylestradiol, which increase the risk of venous and arterial thromboembolism, consequently increase the risk of transient ischemic attack, cerebrovascular accident (stroke), and myocardial infarction (heart attack), and result in substantial increases in cardiovascular mortality.[33][34] It is thought that the relatively minimal cardiovascular toxicity of parenteral forms of estradiol, like PEP and high-dose transdermal estradiol patches,[38] is due to their absence of effect on hepatic coagulation factors.[33][34]
Overdose
Acute toxicity studies have not indicated a risk of acute side effects with overdose of PEP.[1] The median lethal dose (LD50) of PEP in mice is approximately 700 mg/kg.[39] PEP has been administered in total amounts of 2,000 to 3,000 mg over several months in patients with cancer without toxicity observed.[39] The most likely sign of overdose is reversible feminization, namely gynecomastia.[1] Other symptoms of estrogen overdosage may include nausea, vomiting, bloating, increased weight, water retention, breast tenderness, vaginal discharge, heavy legs, and leg cramps.[28] These side effects can be diminished by reducing the estrogen dosage.[28] There is no specific antidote for overdose of PEP.[1] Treatment of PEP overdose should be based on symptoms.[1]
Interactions
Known potential interactions of PEP are mostly the same as those of estradiol and include:[1]
- Cytochrome P450 inhibitors, especially of CYP3A4, can reduce the metabolism of estradiol and thereby increase estradiol levels; examples include anti-infectives (e.g., erythromycin, clarithromycin, ketoconazole, itraconazole), cimetidine, and grapefruit juice[1][40]
- Cytochrome P450 inducers, especially of CYP3A4, can induce the metabolism of estradiol and thereby decrease estradiol levels; examples include anticonvulsants (e.g., phenobarbital, carbamazepine, phenytoin), anti-infectives (rifampicin, rifabutin, nevirapine, and efavirenz), and St. John's wort; in addition, while ritonavir and nelfinavir are known as strong inhibitors, they have an inducing effect in combination with steroid hormones[1]
- Certain antibiotics (e.g., ampicillin, tetracyclines) may decrease estradiol levels by limiting enterohepatic recirculation of estradiol[1]
- Paracetamol (acetaminophen), certain beta blockers (e.g., metoprolol), and some benzodiazepines may increase the effects of PEP[1]
- The coagulation-promoting effects of PEP may enhance those of aminocaproic acid[1]
- Polystyrene phosphate can reduce the effects of anticoagulants[1]
- Estrogens increase thyroxine-binding globulin levels and may result in increased total thyroid hormone levels (but not free thyroid hormone levels in euthyroid individuals);[41] interpretation of thyroid function tests should consider this[1]
- Due to reduced glucose tolerance, there may be an influence on need for insulin or oral antidiabetic medications[1]
- PEP may impair the effects of fibrates (e.g., bezafibrate) and certain nonsteroidal anti-inflammatory drugs (e.g., phenazone)[1]
- Concurrent use of hepatotoxic medications, especially dantrolene, may increase the risk of hepatotoxicity[1]
- Phosphatase inhibitors like levamisole may inhibit the cleavage of PEP into estradiol
Interactions with PEP may be less than with oral estrogens due to the lack of the first-pass through the liver.[1]
Pharmacology
Pharmacodynamics
PEP is an estradiol ester in the form of a polymer and is an extremely long-lasting prodrug of estradiol.[2][7][5][6] As such, it is an estrogen, or an agonist of the estrogen receptors.[2][6][5] PEP has antigonadotropic and functional antiandrogenic effects due to its estrogenic activity.[35] A single repeat unit of PEP, corresponding to estradiol phosphate (minus OH2), is of about 23% higher molecular weight than estradiol due to the presence of its C17β phosphate ester.[42][15] Because PEP is a prodrug of estradiol, it is considered to be a natural and bioidentical form of estrogen.[5]
PEP is a strong inhibitor of several enzymes, including acid phosphatase, alkaline phosphatase, and hyaluronidase, in vitro.[43][44][45] In light of the fact that phosphatases, which cleave PEP into estradiol and phosphoric acid, are present in most tissues in the body, it has been said that the long elimination half-life and slow release of PEP are somewhat surprising.[46] It is thought that PEP may inhibit its own metabolism.[46]
| Estrogen | Form | Dose (mg) | Duration by dose (mg) | ||
|---|---|---|---|---|---|
| EPD | CICD | ||||
| Estradiol | Aq. soln. | ? | – | <1 d | |
| Oil soln. | 40–60 | – | 1–2 ≈ 1–2 d | ||
| Aq. susp. | ? | 3.5 | 0.5–2 ≈ 2–7 d; 3.5 ≈ >5 d | ||
| Microsph. | ? | – | 1 ≈ 30 d | ||
| Estradiol benzoate | Oil soln. | 25–35 | – | 1.66 ≈ 2–3 d; 5 ≈ 3–6 d | |
| Aq. susp. | 20 | – | 10 ≈ 16–21 d | ||
| Emulsion | ? | – | 10 ≈ 14–21 d | ||
| Estradiol dipropionate | Oil soln. | 25–30 | – | 5 ≈ 5–8 d | |
| Estradiol valerate | Oil soln. | 20–30 | 5 | 5 ≈ 7–8 d; 10 ≈ 10–14 d; 40 ≈ 14–21 d; 100 ≈ 21–28 d | |
| Estradiol benz. butyrate | Oil soln. | ? | 10 | 10 ≈ 21 d | |
| Estradiol cypionate | Oil soln. | 20–30 | – | 5 ≈ 11–14 d | |
| Aq. susp. | ? | 5 | 5 ≈ 14–24 d | ||
| Estradiol enanthate | Oil soln. | ? | 5–10 | 10 ≈ 20–30 d | |
| Estradiol dienanthate | Oil soln. | ? | – | 7.5 ≈ >40 d | |
| Estradiol undecylate | Oil soln. | ? | – | 10–20 ≈ 40–60 d; 25–50 ≈ 60–120 d | |
| Polyestradiol phosphate | Aq. soln. | 40–60 | – | 40 ≈ 30 d; 80 ≈ 60 d; 160 ≈ 120 d | |
| Estrone | Oil soln. | ? | – | 1–2 ≈ 2–3 d | |
| Aq. susp. | ? | – | 0.1–2 ≈ 2–7 d | ||
| Estriol | Oil soln. | ? | – | 1–2 ≈ 1–4 d | |
| Polyestriol phosphate | Aq. soln. | ? | – | 50 ≈ 30 d; 80 ≈ 60 d | |
Notes and sources
Notes: All aqueous suspensions are of microcrystalline particle size. Estradiol production during the menstrual cycle is 30–640 µg/d (6.4–8.6 mg total per month or cycle). The vaginal epithelium maturation dosage of estradiol benzoate or estradiol valerate has been reported as 5 to 7 mg/week. An effective ovulation-inhibiting dose of estradiol undecylate is 20–30 mg/month. Sources: See template. | |||||
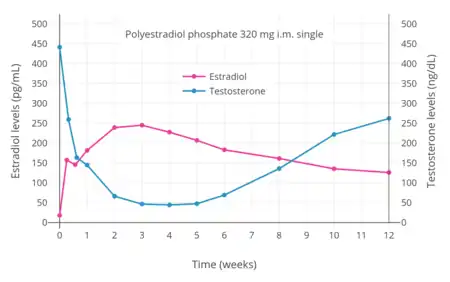
- Hormone levels with polyestradiol phosphate by intramuscular injection
Antigonadotropic effects
PEP has antigonadotropic effects due to its estrogenic activity.[36] It has been found to suppress testosterone levels in men by 55%, 75%, and 85% at intramuscular dosages of 80, 160, and 240 mg every 4 weeks, respectively.[46] A single intramuscular injection of 320 mg PEP in men has been found to suppress testosterone levels to within the castrate range (< 50 ng/dL) within 3 weeks.[7] This was associated with circulating estradiol levels of just over 200 pg/mL.[35] The suppression of testosterone levels that can be achieved with PEP is equal to that with orchiectomy.[48] However, to achieve such concentrations of testosterone, which are about 15 ng/dL on average, higher concentrations of estradiol of around 500 pg/mL were necessary.[35][48][49] This was associated with a dosage of intramuscular 320 mg PEP every four weeks and occurred by 90 days of treatment.[35] However, 240 mg PEP every four weeks has also been reported to eventually suppress testosterone levels in the castrate range.[50][51]
Mechanism of action in prostate cancer
The growth of prostate cancer is generally stimulated by dihydrotestosterone (DHT), and unless the cancer is castration-resistant, it can be treated by depriving it of androgens. Estradiol produces its therapeutic benefits mainly via exertion of negative feedback on the hypothalamic–pituitary–gonadal axis.[36][46][7] This blocks the secretion of luteinizing hormone, which in turn reduces testosterone production in the Leydig cells of the testes.[36][46][7] Estradiol also decreases the free percentage of testosterone by increasing sex hormone-binding globulin (SHBG) levels.[7] In addition, it exhibits direct cytotoxicity on prostate cancer cells.[52][20]
Differences from other estrogens
| Factors | Oral estrogensa | Parenteral estrogensb |
|---|---|---|
| Factor VII | Increases | No change |
| Factor VIII activity | Increases | No change |
| Antithrombin III activity | Decreases | Decreases |
| Prothrombin fragment 1+2 | Increases | No change |
| Activated protein C resistance | Increases | No change |
| Fibrinogen | Decreases | No change |
| Footnotes: a = E.g., DES, EE. b = PEP. Sources: [34] | ||
Estrogens have effects on liver protein synthesis, including on the synthesis of plasma proteins, coagulation factors, lipoproteins, and triglycerides.[48] These effects can result in an increased risk of thromboembolic and cardiovascular complications, which in turn can result in increased mortality.[48] Studies have found a markedly increased 5-year risk of cardiovascular mortality of 14 to 26% in men treated with oral synthetic estrogens like ethinylestradiol and diethylstilbestrol for prostate cancer.[48] However, whereas oral synthetic estrogens have a strong influence on liver protein synthesis, the effects of parenteral bioidentical estrogens like PEP on liver protein synthesis are comparatively very weak or even completely abolished.[48] This is because the first-pass through the liver with oral administration is avoided and because bioidentical estrogens are efficiently inactivated in the liver.[48] In accordance, PEP has minimal effect on the liver at a dosage of up to at least 240 mg/month.[53]
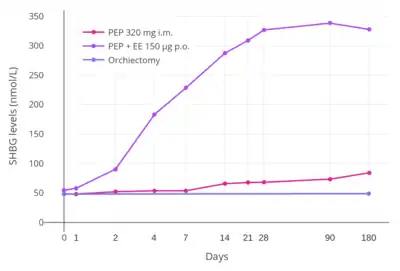
A study found that whereas 320 mg/month intramuscular PEP increased SHBG levels to 166% in men with prostate cancer, the combination of 80 mg/month intramuscular polyestradiol phosphate and 150 µg/day oral ethinylestradiol increased levels of SHBG to 617%, an almost 8-fold difference in increase and almost 4-fold difference in absolute levels between the two treatment regimens.[35][7][54] In addition, whereas there were no cardiovascular complications in the PEP-only group, there was a 25% incidence of cardiovascular complications over the course of a year in the group that was also treated with ethinylestradiol.[7] Another study found no change in levels of coagulation factor VII, a protein of particular importance in the cardiovascular side effects of estrogens, with 240 mg/month intramuscular PEP.[53] These findings demonstrate the enormous impact of synthetic oral estrogens like ethinylestradiol on liver protein production relative to parenteral bioidentical forms of estrogen like PEP.[7]
Originally, PEP was typically used at a dosage of 80 mg per month in combination with 150 μg per day oral ethinylestradiol in the treatment of prostate cancer.[46][55] This combination was found to produce a considerable incidence of cardiovascular toxicity,[48] and this toxicity was inappropriately attributed to PEP in some publications.[56] Subsequent research has shown that the toxicity is not due to PEP but rather to the ethinylestradiol component.[57][46][48]
A study found that therapy with intramuscular PEP resulting in estradiol levels of around 400 pg/mL in men with prostate cancer did not affect growth hormone or insulin-like growth factor 1 levels, whereas the addition of oral ethinylestradiol significantly increased growth hormone levels and decreased insulin-like growth factor 1 levels.[58][59]
Pharmacokinetics
PEP has a very long duration and is given by intramuscular injection once every 4 weeks.[35] In men, an initial intramuscular injection of PEP results in a rapid rise in estradiol levels measured at 24 hours followed by a slow and gradual further increase in levels up until at least day 28 (the time of the next injection).[35] Subsequent injections result in a progressive and considerable accumulation in estradiol levels up to at least 6 months.[35] The mean elimination half-life of PEP has been found to be 70 days (10 weeks) with a single 320 mg intramuscular dose of the medication.[7] The tmax (time to maximal concentrations) for estradiol was about 16 days.[7] PEP has a duration of approximately 1 month with a single dose of 40 mg, 2 months with 80 mg, and 4 months with 160 mg.[60]</ref>[61][62][63][39]
PEP reaches the bloodstream within hours after an injection (90% after 24 hours), where it circulates, and is accumulated in the reticuloendothelial system.[52] Estradiol is then cleaved from the polymer by phosphatases, although very slowly.[64] Levels of estradiol in men with intramuscular injections of PEP once every 4 weeks were about 350 pg/mL with 160 mg, 450 pg/mL with 240 mg, and almost 700 pg/mL with 320 mg, all measured after 6 months of treatment.[35] With monthly injections, steady-state estradiol concentrations are reached after 6 to 12 months.[52] Estradiol is metabolized primarily in the liver by CYP3A4 and other cytochrome P450 enzymes, and is metabolized to a lesser extent in extrahepatic tissues.[20][1] The metabolites are mainly excreted in urine via the kidneys.[1]
Early studies found that a dosage of 80 mg PEP every 4 weeks rapidly produced relatively high mean estradiol levels of about 400 to 800 pg/mL.[65] These levels are similar to those of 100 mg estradiol undecylate every month, which has been found to produce estradiol levels of around 500 to 600 pg/mL.[66][67] As a result, it has previously been said that 80 mg PEP per month and 100 mg estradiol undecylate per month are roughly equivalent.[68][69][7] However, subsequent studies showed that this dosage of PEP actually achieves much lower estradiol levels than originally demonstrated.[35]
Chemistry
PEP is a synthetic estrane steroid and the C17β phosphoric acid (phosphate) ester of estradiol (estradiol 17β-phosphate) in the form of a polymer.[70][15][46][71] It is also known as estradiol polymer with phosphoric acid or as estradiol 17β-phosphate polymer, as well as estra-1,3,5(10)-triene-3,17β-diol 17β-phosphate polymer.[15][70][46][71] It has been determined via ultracentrifugation that the mean molecular weight of PEP corresponds to a chain length of approximately 13 repeat units of estradiol 17β-phosphate.[46] PEP is closely related to polyestriol phosphate (Gynäsan, Klimadurin, Triodurin) and polytestosterone phloretin phosphate (never commercialized), which are estriol and testosterone esters in the forms of polymers, respectively.[60]A. Labhart (6 December 2012). Clinical Endocrinology: Theory and Practice. Springer Science & Business Media. pp. 551–. ISBN 978-3-642-96158-8. The polymer of estradiol or estriol and phosphoric acid has an excellent depot action when given intramuscularly (polyestriol phosphate or polyestradiol phosphate) (Table 16). Phosphoric acid combines with the estrogen molecule at C3 and C17 to form a macromolecule. The compound is stored in the liver and spleen where the estrogen is steadily released by splitting off of the phosphate portion due to the action of alkaline phosphatase. [...] Conjugated estrogens and polyestriol and estradiol phosphate can also be given intravenously in an aqueous solution. Intravenous administration of ovarian hormones offers no advantages, however, and therefore has no practical significance. [...] The following duarations of action have been obtained with a single administration (WlED, 1954; LAURITZEN, 1968): [...] 50 mg polyestradiol phosphate ~ 1 month; 50 mg polyestriol phosphate ~ 1 month; 80 mg polyestriol phosphate ~ 2 months.
</ref>[72] It is also related to polydiethylstilbestrol phosphate (never commercialized), a diethylstilbestrol ester in the form of a polymer.[73]
| Estrogen | Structure | Ester(s) | Relative mol. weight | Relative E2 contentb | logPc | ||||
|---|---|---|---|---|---|---|---|---|---|
| Position(s) | Moiet(ies) | Type | Lengtha | ||||||
| Estradiol | – | – | – | – | 1.00 | 1.00 | 4.0 | ||
| Estradiol acetate | C3 | Ethanoic acid | Straight-chain fatty acid | 2 | 1.15 | 0.87 | 4.2 | ||
| Estradiol benzoate | C3 | Benzenecarboxylic acid | Aromatic fatty acid | – (~4–5) | 1.38 | 0.72 | 4.7 | ||
| Estradiol dipropionate | C3, C17β | Propanoic acid (×2) | Straight-chain fatty acid | 3 (×2) | 1.41 | 0.71 | 4.9 | ||
| Estradiol valerate | C17β | Pentanoic acid | Straight-chain fatty acid | 5 | 1.31 | 0.76 | 5.6–6.3 | ||
| Estradiol benzoate butyrate | C3, C17β | Benzoic acid, butyric acid | Mixed fatty acid | – (~6, 2) | 1.64 | 0.61 | 6.3 | ||
| Estradiol cypionate | C17β | Cyclopentylpropanoic acid | Aromatic fatty acid | – (~6) | 1.46 | 0.69 | 6.9 | ||
| Estradiol enanthate | C17β | Heptanoic acid | Straight-chain fatty acid | 7 | 1.41 | 0.71 | 6.7–7.3 | ||
| Estradiol dienanthate | C3, C17β | Heptanoic acid (×2) | Straight-chain fatty acid | 7 (×2) | 1.82 | 0.55 | 8.1–10.4 | ||
| Estradiol undecylate | C17β | Undecanoic acid | Straight-chain fatty acid | 11 | 1.62 | 0.62 | 9.2–9.8 | ||
| Estradiol stearate | C17β | Octadecanoic acid | Straight-chain fatty acid | 18 | 1.98 | 0.51 | 12.2–12.4 | ||
| Estradiol distearate | C3, C17β | Octadecanoic acid (×2) | Straight-chain fatty acid | 18 (×2) | 2.96 | 0.34 | 20.2 | ||
| Estradiol sulfate | C3 | Sulfuric acid | Water-soluble conjugate | – | 1.29 | 0.77 | 0.3–3.8 | ||
| Estradiol glucuronide | C17β | Glucuronic acid | Water-soluble conjugate | – | 1.65 | 0.61 | 2.1–2.7 | ||
| Estramustine phosphated | C3, C17β | Normustine, phosphoric acid | Water-soluble conjugate | – | 1.91 | 0.52 | 2.9–5.0 | ||
| Polyestradiol phosphatee | C3–C17β | Phosphoric acid | Water-soluble conjugate | – | 1.23f | 0.81f | 2.9g | ||
| Footnotes: a = Length of ester in carbon atoms for straight-chain fatty acids or approximate length of ester in carbon atoms for aromatic fatty acids. b = Relative estradiol content by weight (i.e., relative estrogenic exposure). c = Experimental or predicted octanol/water partition coefficient (i.e., lipophilicity/hydrophobicity). Retrieved from PubChem, ChemSpider, and DrugBank. d = Also known as estradiol normustine phosphate. e = Polymer of estradiol phosphate (~13 repeat units). f = Relative molecular weight or estradiol content per repeat unit. g = logP of repeat unit (i.e., estradiol phosphate). Sources: See individual articles. | |||||||||
Solubility
PEP is of very low solubility in water, acetone, chloroform, dioxane, and ethanol, but dissolves readily in bases, especially in aqueous pyridine.[52]
Synthesis
Like polyphosphates of polyphenols, PEP can be prepared from the monomer (in this case estradiol) and phosphoryl chloride. The latter reacts with both the phenolic hydroxyl group in position 3 and the aliphatic one in position 17β. The molecular mass of the resulting polymer can be controlled by interrupting the reaction after a given time: the longer the reaction is allowed to continue, the higher the mass.[64][74]
History
Pharmacological experiments on estradiol phosphates conducted around 1950 gave rise to the hypothesis that estradiol 3,17β-diphosphate acted as an inhibitor of kidney alkaline phosphatase.[64] When the same scientists wanted to synthesize simple phosphates of phloretin, a compound found in apple tree leaves,[75] they accidentally created a polymer instead.[74] This was later shown to exhibit the same anti-phosphatase properties as estradiol diphosphate, and so it was hypothesized that the original finding was due to contamination with estradiol phosphate polymers.[64] Consequently, these polymers were studied in more detail, which resulted in the development of PEP as early as 1953[12] and its subsequent introduction for medical use in 1957 in the United States.[13][14][76]
Society and culture
Generic names
Polyestradiol phosphate is the generic name of the drug and its INN and BAN.[15][70][42] It is also known by its developmental code name Leo-114.[15][42]
Availability
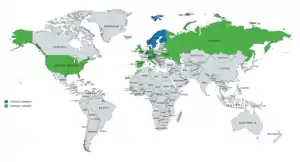
PEP has been marketed in the United States and widely throughout Europe, including in Austria, Belgium, the Czech Republic, Denmark, Finland, Germany, Italy, the Netherlands, Norway, Russia, Spain, Sweden, Switzerland, Ukraine, and the United Kingdom.[15][26][1][77][78][14] It is no longer available in the United States and possibly certain other countries however,[13][16] but is still known to be marketed in Austria, Belgium, Denmark, Finland, the Netherlands, Norway, Sweden, and Switzerland.[42][77][78][1]
Research
PEP has been studied as a means of hormonal breast enhancement in women.[79]
A combination of PEP and medroxyprogesterone acetate was studied in women as a long-lasting combined injectable contraceptive for use by intramuscular injection once every three months.[80][81][82]
References
- "Archived copy". Archived from the original on 2018-01-02. Retrieved 2018-01-01.CS1 maint: archived copy as title (link)
- Mikkola A, Ruutu M, Aro J, Rannikko S, Salo J (1999). "The role of parenteral polyestradiol phosphate in the treatment of advanced prostatic cancer on the threshold of the new millennium". Ann Chir Gynaecol. 88 (1): 18–21. ISSN 0355-9521. PMID 10230677.
Orchiectomy and estrogens have been used for over 50 years in the treatment of advanced prostatic cancer. Although orchiectomy is a simple procedure, it may cause psychological stress. Oral estrogen therapy is as effective as orchiectomy in terms of cancer inhibitory effect, but its acceptance as primary hormonal treatment is overshadowed by an increased risk of cardiovascular complications. Parenteral estrogen, polyestradiol phosphate (PEP), is effective, but also associated with cardiovascular complications, although to a lesser extent. During the last 20 years, well tolerated luteinizing hormone releasing hormone (LHRH) analogues have been replacing orchiectomy and estrogens. Efforts have been made to increase the efficacy of the treatment by adding antiandrogens to LHRH analogues and also to orchiectomy (combined androgen blockade, CAB). However, the efficacy of LHRH analogues and CAB has not proved to be superior to that of simple orchiectomy and, moreover, they are expensive treatment modalities. Orchiectomy and LHRH analogues are associated with negative effects on bone mass and may cause osteoporosis, whereas PEP treatment has an opposite effect. Parenteral polyestradiol phosphate is still a cheap potential treatment for advanced prostatic cancer, but further studies should be conducted to establish its future role, e.g. combining acetylsalicylic acid to prevent cardiovascular complications.
- Stanczyk, Frank Z.; Archer, David F.; Bhavnani, Bhagu R. (2013). "Ethinyl estradiol and 17β-estradiol in combined oral contraceptives: pharmacokinetics, pharmacodynamics and risk assessment". Contraception. 87 (6): 706–727. doi:10.1016/j.contraception.2012.12.011. ISSN 0010-7824. PMID 23375353.
- Tommaso Falcone; William W. Hurd (2007). Clinical Reproductive Medicine and Surgery. Elsevier Health Sciences. pp. 22, 362, 388. ISBN 978-0-323-03309-1.
- Michael Oettel; Ekkehard Schillinger (6 December 2012). Estrogens and Antiestrogens II: Pharmacology and Clinical Application of Estrogens and Antiestrogen. Springer Science & Business Media. p. 261,544. ISBN 978-3-642-60107-1.
Natural estrogens considered here include: [...] Esters of 17β-estradiol, such as estradiol valerate, estradiol benzoate and estradiol cypionate. Esterification aims at either better absorption after oral administration or a sustained release from the depot after intramuscular administration. During absorption, the esters are cleaved by endogenous esterases and the pharmacologically active 17β-estradiol is released; therefore, the esters are considered as natural estrogens.
- Kuhl H (2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration" (PDF). Climacteric. 8 Suppl 1: 3–63. doi:10.1080/13697130500148875. PMID 16112947. S2CID 24616324.
- Stege R, Gunnarsson PO, Johansson CJ, Olsson P, Pousette A, Carlström K (May 1996). "Pharmacokinetics and testosterone suppression of a single dose of polyestradiol phosphate (Estradurin) in prostatic cancer patients". Prostate. 28 (5): 307–10. doi:10.1002/(SICI)1097-0045(199605)28:5<307::AID-PROS6>3.0.CO;2-8. PMID 8610057.
- Düsterberg B, Nishino Y (1982). "Pharmacokinetic and pharmacological features of oestradiol valerate". Maturitas. 4 (4): 315–24. doi:10.1016/0378-5122(82)90064-0. PMID 7169965.
- Stege R, Carlström K, Hedlund PO, Pousette A, von Schoultz B, Henriksson P (September 1995). "Intramuskuläres Depotöstrogen (Estradurin) in der Behandlung von Patienten mit Prostatakarzinom. Historische Aspekte, Wirkungsmechanismus, Resultate und aktueller klinischer Stand" [Intramuscular depot estrogens (Estradurin) in treatment of patients with prostate carcinoma. Historical aspects, mechanism of action, results and current clinical status]. Urologe A (in German). 34 (5): 398–403. ISSN 0340-2592. PMID 7483157.
More than 50 years ago, orally given estrogen was already used in the treatment of prostate cancer. Due to cardiovascular side-effects with a high morbidity of 25%, this treatment has not become standard. Recent investigations show that parenteral application reduces the risk of cardiovascular side-effects, because it avoids the first passage through the liver with high concentrations of estrogen which normally occur after oral application. Therefore, an increased synthesis of so-called "steroid-sensitive" liver proteins, such as coagulation factors (especially factor VII) can be avoided. This newer parenteral estrogen application shows encouraging results of a cheap and effective hormonal therapy with a low rate of side-effects in patients with prostate cancer.
- Mikkola, A; Aro, J; Rannikko, S; Ruutu, M; Finnprostate, Group (March 2007). "Ten-year survival and cardiovascular mortality in patients with advanced prostate cancer primarily treated by intramuscular polyestradiol phosphate or orchiectomy". Prostate. 67 (4): 447–55. doi:10.1002/pros.20547. PMID 17219379. S2CID 20549248.
- Urdl, W. (2009). "Behandlungsgrundsätze bei Transsexualität" [Therapeutic principles in transsexualism]. Gynäkologische Endokrinologie (in German). 7 (3): 153–160. doi:10.1007/s10304-009-0314-9. ISSN 1610-2894. S2CID 8001811.
- Steinbach T, Wurm FR (2015). "Poly(phosphoester)s: A New Platform for Degradable Polymers". Angew. Chem. Int. Ed. Engl. 54 (21): 6098–108. doi:10.1002/anie.201500147. PMID 25951459.
- "Drugs@FDA: FDA Approved Drug Products: Estradurin". United States Food and Drug Administration. Retrieved 24 June 2018.
- William Andrew Publishing (22 October 2013). Pharmaceutical Manufacturing Encyclopedia. Elsevier. pp. 2934–2935. ISBN 978-0-8155-1856-3.
- Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 856–. ISBN 978-3-88763-075-1.
- Mosby (11 February 2009). Mosby's Pocket Dictionary of Medicine, Nursing & Health Professions. Elsevier Health Sciences. pp. 3672–. ISBN 978-0-323-06604-4.
- Ostrowski MJ, Jackson AW (1979). "Polyestradiol phosphate: a preliminary evaluation of its effect on breast carcinoma". Cancer Treat Rep. 63 (11–12): 1803–7. PMID 393380.
- Brunner N, Spang-Thomsen M, Cullen K (1996). "The T61 human breast cancer xenograft: an experimental model of estrogen therapy of breast cancer". Breast Cancer Res. Treat. 39 (1): 87–92. doi:10.1007/bf01806080. PMID 8738608. S2CID 27430232.
[...] In a study with parenteral estrogen therapy of patients with metastatic breast cancer, 14/24 patients obtained an objective response (including patients with stable disease >6 months) [13]. The only side effect reported was bleeding from a hyperplastic endometrium.
- Arver DS (2015). "Transsexualism, könsdysfori" [Transsexualism, gender dysphoria]. Retrieved 2018-11-12.
- W, Jasek, ed. (2007). Austria-Codex (in German) (62nd ed.). Vienna: Österreichischer Apothekerverlag. pp. 2992–4. ISBN 978-3-85200-181-4.
- Sayed Y, Taxel P (2003). "The use of estrogen therapy in men". Curr Opin Pharmacol. 3 (6): 650–4. doi:10.1016/j.coph.2003.07.004. PMID 14644018.
- Hedlund PO, Henriksson P (2000). "Parenteral estrogen versus total androgen ablation in the treatment of advanced prostate carcinoma: effects on overall survival and cardiovascular mortality. The Scandinavian Prostatic Cancer Group (SPCG)-5 Trial Study". Urology. 55 (3): 328–33. doi:10.1016/s0090-4295(99)00580-4. PMID 10699602.
- Deepinder F, Braunstein GD (2012). "Drug-induced gynecomastia: an evidence-based review". Expert Opinion on Drug Safety. 11 (5): 779–95. doi:10.1517/14740338.2012.712109. PMID 22862307. S2CID 22938364.
Treatment with estrogen has the highest incidence of gynecomastia, at 40 – 80%, anti-androgens, including flutamide, bicalutamide and nilutamide, are next, with a 40 – 70% incidence, followed by GnRH analogs (goserelin, leuprorelin) and combined androgen deprivation, both with incidences of 13% each.
- Norman G, Dean ME, Langley RE, Hodges ZC, Ritchie G, Parmar MK, Sydes MR, Abel P, Eastwood AJ (February 2008). "Parenteral oestrogen in the treatment of prostate cancer: a systematic review". Br. J. Cancer. 98 (4): 697–707. doi:10.1038/sj.bjc.6604230. PMC 2259178. PMID 18268497.
- Schlatterer K, von Werder K, Stalla GK (1996). "Multistep treatment concept of transsexual patients". Exp. Clin. Endocrinol. Diabetes. 104 (6): 413–9. doi:10.1055/s-0029-1211479. PMID 9021341.
- Muller (19 June 1998). European Drug Index: European Drug Registrations, Fourth Edition. CRC Press. pp. 455–. ISBN 978-3-7692-2114-5.
- https://web.archive.org/web/*/https://www.pfizer.com/sites/default/files/products/material_safety_data/ESTRADURINE.pdf
- Lauritzen C (September 1990). "Clinical use of oestrogens and progestogens". Maturitas. 12 (3): 199–214. doi:10.1016/0378-5122(90)90004-P. PMID 2215269.
- Christian Lauritzen; John W. W. Studd (22 June 2005). Current Management of the Menopause. CRC Press. pp. 95–98, 488. ISBN 978-0-203-48612-2.
- Laurtizen, Christian (2001). "Hormone Substitution Before, During and After Menopause" (PDF). In Fisch, Franz H. (ed.). Menopause – Andropause: Hormone Replacement Therapy Through the Ages. Krause & Pachernegg: Gablitz. pp. 67–88. ISBN 978-3-901299-34-6.
- Midwinter, Audrey (1976). "Contraindications to estrogen therapy and management of the menopausal syndrome in these cases". In Campbell, Stuart (ed.). The Management of the Menopause & Post-Menopausal Years: The Proceedings of the International Symposium held in London 24–26 November 1975 Arranged by the Institute of Obstetrics and Gynaecology, The University of London. MTP Press Limited. pp. 377–382. doi:10.1007/978-94-011-6165-7_33. ISBN 978-94-011-6167-1.
- Burkhard Helpap; Herbert Rübben (12 March 2013). Prostatakarzinom — Pathologie, Praxis und Klinik: Pathologie, Praxis und Klinik. Springer-Verlag. pp. 126–. ISBN 978-3-642-72110-6.
- Ockrim J, Lalani EN, Abel P (October 2006). "Therapy Insight: parenteral estrogen treatment for prostate cancer—a new dawn for an old therapy". Nat Clin Pract Oncol. 3 (10): 552–63. doi:10.1038/ncponc0602. PMID 17019433. S2CID 6847203.
- Lycette JL, Bland LB, Garzotto M, Beer TM (December 2006). "Parenteral estrogens for prostate cancer: can a new route of administration overcome old toxicities?". Clin Genitourin Cancer. 5 (3): 198–205. doi:10.3816/CGC.2006.n.037. PMID 17239273.
- Stege R, Carlström K, Collste L, Eriksson A, Henriksson P, Pousette A (1988). "Single drug polyestradiol phosphate therapy in prostatic cancer". Am. J. Clin. Oncol. 11 Suppl 2: S101–3. doi:10.1097/00000421-198801102-00024. PMID 3242384. S2CID 32650111.
- Waun Ki Hong; James F. Holland (2010). Holland-Frei Cancer Medicine 8. PMPH-USA. pp. 753–. ISBN 978-1-60795-014-1.
- Russell N, Cheung A, Grossmann M (August 2017). "Estradiol for the mitigation of adverse effects of androgen deprivation therapy". Endocr. Relat. Cancer. 24 (8): R297–R313. doi:10.1530/ERC-17-0153. PMID 28667081.
- Langley RE, Cafferty FH, Alhasso AA, Rosen SD, Sundaram SK, Freeman SC, Pollock P, Jinks RC, Godsland IF, Kockelbergh R, Clarke NW, Kynaston HG, Parmar MK, Abel PD (April 2013). "Cardiovascular outcomes in patients with locally advanced and metastatic prostate cancer treated with luteinising-hormone-releasing-hormone agonists or transdermal oestrogen: the randomised, phase 2 MRC PATCH trial (PR09)". Lancet Oncol. 14 (4): 306–16. doi:10.1016/S1470-2045(13)70025-1. PMC 3620898. PMID 23465742.
- Diczfalsuy E, Westman A (April 1956). "Urinary excretion of natural oestrogens in oophorectomized women treated with polyoestradiol phosphate (PEP)". Acta Endocrinol. 21 (4): 321–36. doi:10.1530/acta.0.0210321. PMID 13312990.
- Cheng ZN, Shu Y, Liu ZQ, Wang LS, Ou-Yang DS, Zhou HH (2001). "Role of cytochrome P450 in estradiol metabolism in vitro". Acta Pharmacol. Sin. 22 (2): 148–54. PMID 11741520.
- Mazer NA (2004). "Interaction of estrogen therapy and thyroid hormone replacement in postmenopausal women". Thyroid. 14 Suppl 1: S27–34. doi:10.1089/105072504323024561. PMID 15142374.
- https://www.drugs.com/international/polyestradiol-phosphate.html
- Lindstedt E (1980). "Polyestradiol phosphate and ethinyl estradiol in treatment of prostatic carcinoma". Scand J Urol Nephrol Suppl. 55: 95–7. PMID 6938044.
Polyestradiol phosphate is a polymeric ester of estradiol -17 beta and phosphoric acid. The large molecule has very weak estrogenic properties but is a strong inhibitor of several enzymes, e.g. acid and alkaline phosphatases and hyaluronidase.
- Steven FS, Griffin MM (1982). "Inhibition of thrombin cleavage of fibrinogen by polyestradiol phosphate; interaction with the crucial arginine residues in fibrinogen required for enzymic cleavage". Int. J. Biochem. 14 (8): 699–700. doi:10.1016/0020-711X(82)90004-0. PMID 7117668.
Polyestradiol phosphate (PEP) has been demonstrated to have inhibitory activity against hyaluronidase, acid phosphatase and alkaline phosphatase (Fernö et al., 1958).
- Fernö O, Fex H, Högberg B, Linderot T, Veige S (1958). "High Molecular Weight Enzyme Inhibitors. 3. Polyestradiol Phosphate (PEP), a Long-acting Estrogen". Acta Chemica Scandinavica. 12 (8): 1675–1689. doi:10.3891/acta.chem.scand.12-1675.
- Gunnarsson PO, Norlén BJ (1988). "Clinical pharmacology of polyestradiol phosphate". Prostate. 13 (4): 299–304. doi:10.1002/pros.2990130405. PMID 3217277. S2CID 33063805.
- Stege R, Carlström K, Collste L, Eriksson A, Henriksson P, Pousette A (1988). "Single drug polyestradiol phosphate therapy in prostatic cancer". Am. J. Clin. Oncol. 11 Suppl 2: S101–3. doi:10.1097/00000421-198801102-00024. PMID 3242384. S2CID 32650111.
- von Schoultz B, Carlström K, Collste L, Eriksson A, Henriksson P, Pousette A, Stege R (1989). "Estrogen therapy and liver function—metabolic effects of oral and parenteral administration". Prostate. 14 (4): 389–95. doi:10.1002/pros.2990140410. PMID 2664738. S2CID 21510744.
- Gokhan Ozyigit; Ugur Selek (1 August 2017). Principles and Practice of Urooncology: Radiotherapy, Surgery and Systemic Therapy. Springer. pp. 334–. ISBN 978-3-319-56114-1.
The castrate level was defined as testosterone being less than 50 ng/dL (1.7 nmol/L), many years ago. However contemporary laboratory testing methods showed that the mean value after surgical castration is 15 ng/dL [1]. Thus, recently the level is defined as being less than 20 ng/dL (1 nmol/L).
- Mikkola AK, Ruutu ML, Aro JL, Rannikko SA, Salo JO (July 1998). "Parenteral polyoestradiol phosphate vs orchidectomy in the treatment of advanced prostatic cancer. Efficacy and cardiovascular complications: a 2-year follow-up report of a national, prospective prostatic cancer study. Finnprostate Group". Br J Urol. 82 (1): 63–8. doi:10.1046/j.1464-410x.1998.00688.x. PMID 9698663.
- Stege, R.; Carlström, K.; Collste, L.; Eriksson, A.; Henriksson, P. (1987). "Single drug polyestradiol phosphate (PEP) therapy in prostatic cancer (CAP)". European Journal of Cancer and Clinical Oncology. 23 (8): 1249. doi:10.1016/0277-5379(87)90236-7. ISSN 0277-5379.
- Dinnendahl, V; Fricke, U, eds. (2010). Arzneistoff-Profile (in German). 4 (23 ed.). Eschborn, Germany: Govi Pharmazeutischer Verlag. ISBN 978-3-7741-98-46-3.
- Henriksson P, Carlström K, Pousette A, Gunnarsson PO, Johansson CJ, Eriksson B, Altersgård-Brorsson AK, Nordle O, Stege R (1999). "Time for revival of estrogens in the treatment of advanced prostatic carcinoma? Pharmacokinetics, and endocrine and clinical effects, of a parenteral estrogen regimen". Prostate. 40 (2): 76–82. doi:10.1002/(sici)1097-0045(19990701)40:2<76::aid-pros2>3.0.co;2-q. PMID 10386467.
- Carlström K, Collste L, Eriksson A, Henriksson P, Pousette A, Stege R, von Schoultz B (1989). "A comparison of androgen status in patients with prostatic cancer treated with oral and/or parenteral estrogens or by orchidectomy". Prostate. 14 (2): 177–82. doi:10.1002/pros.2990140210. PMID 2523531. S2CID 25516937.
- Cox RL, Crawford ED (December 1995). "Estrogens in the treatment of prostate cancer". J. Urol. 154 (6): 1991–8. doi:10.1016/S0022-5347(01)66670-9. PMID 7500443.
- Wenderoth, U. K.; Jacobi, G. H. (1983). "Gonadotropin-releasing hormone analogues for palliation of carcinoma of the prostate". World Journal of Urology. 1 (1): 40–48. doi:10.1007/BF00326861. ISSN 0724-4983. S2CID 23447326.
- Oh WK (September 2002). "The evolving role of estrogen therapy in prostate cancer". Clin Prostate Cancer. 1 (2): 81–9. doi:10.3816/CGC.2002.n.009. PMID 15046698.
- Stege R, Fröhlander N, Carlström K, Pousette A, von Schoultz B (1987). "Steroid-sensitive proteins, growth hormone and somatomedin C in prostatic cancer: effects of parenteral and oral estrogen therapy". Prostate. 10 (4): 333–8. doi:10.1002/pros.2990100407. PMID 2440014. S2CID 36814574.
- von Schoultz B, Carlström K (February 1989). "On the regulation of sex-hormone-binding globulin--a challenge of an old dogma and outlines of an alternative mechanism". J. Steroid Biochem. 32 (2): 327–34. doi:10.1016/0022-4731(89)90272-0. PMID 2646476.
- A. Labhart (6 December 2012). Clinical Endocrinology: Theory and Practice. Springer Science & Business Media. pp. 551–553. ISBN 978-3-642-96158-8.
- Karl Knörr; Henriette Knörr-Gärtner; Fritz K. Beller; Christian Lauritzen (8 March 2013). Lehrbuch der Geburtshilfe und Gynäkologie: Physiologie und Pathologie der Reproduktion. Springer-Verlag. pp. 508–. ISBN 978-3-662-00526-2.
- Karl Knörr; Fritz K. Beller; Christian Lauritzen (17 April 2013). Lehrbuch der Gynäkologie. Springer-Verlag. p. 213. ISBN 978-3-662-00942-0.
- Brambilla F, Berczeller PH, Epstein JA, Blatt MH, Kupperman HS (1961). "Experiences with the Use of Polyestradiol Phosphate, a Long-acting Estrogen". Obstetrics & Gynecology. 17 (1): 115–118. doi:10.1097/00006250-196101000-00024 (inactive 2021-01-20).CS1 maint: DOI inactive as of January 2021 (link)
- Diczfalusy, E (April 1954). "Poly-estradiol phosphate (PEP); a long-acting water soluble estrogen". Endocrinology. 54 (4): 471–7. doi:10.1210/endo-54-4-471. PMID 13151143.
- Jacobi, GR (1 December 1982). "Experimental Rationale for the Investigation of Antiprolactins as Palliative Treatment for Prostate Cancer". In Jacobi, Günther H; Hohenfellner, Rudolf (eds.). Prostate Cancer. Williams & Wilkins. p. 426. ISBN 978-0-683-04354-9.
- Vermeulen A (1975). "Longacting steroid preparations". Acta Clin Belg. 30 (1): 48–55. doi:10.1080/17843286.1975.11716973. PMID 1231448.
- Jacobi, G.H.; Altwein, J.E. (1979). "Bromocriptin als Palliativtherapie beim fortgeschrittenen Prostatakarzinom:Experimentelles und klinisches Profil eines Medikamentes" [Bromocriptine as Palliative Therapy in Advanced Prostate Cancer: Experimental and Clinical Profile of a Drugjournal=Urologia Internationalis]. Urologia Internationalis. 34 (4): 266–290. doi:10.1159/000280272. ISSN 1423-0399. PMID 89747.
- Jacobi GH, Altwein JE, Kurth KH, Basting R, Hohenfellner R (1980). "Treatment of advanced prostatic cancer with parenteral cyproterone acetate: a phase III randomised trial". Br J Urol. 52 (3): 208–15. doi:10.1111/j.1464-410x.1980.tb02961.x. PMID 7000222.
- P. H. Smith (29 June 2013). Cancer of the Prostate and Kidney. Springer Science & Business Media. pp. 307–. ISBN 978-1-4684-4349-3.
- S. Gangolli (1999). The Dictionary of Substances and Their Effects: O-S. Royal Society of Chemistry. pp. 425–. ISBN 978-0-85404-833-5.
- Johansson, CJ; Gunnarsson, PO (June 2000). "Pharmacodynamic model of testosterone suppression after intramuscular depot estrogen therapy in prostate cancer". Prostate. 44 (1): 26–30. doi:10.1002/1097-0045(20000615)44:1<26::AID-PROS4>3.0.CO;2-P. PMID 10861754.
- US patent 2928849, Högberg Knut Bertil; Fernö Ove Birger & Linderot Torsten Ove Enok et al., "High-molecular weight derivatives of steroids containing hydroxyl groups and method of producing the same", published 15 March 1960, assigned to Leo Ab
- Diczfalusy E, Fernö H, Fex B, Högberg B, Kneip P (1959). "High Molecular Weight Enzyme Inhibitors. IV. Polymeric Phosphates of Synthetic Estrogens" (PDF). Acta Chem. Scand. 13 (5): 1011–1018. doi:10.3891/acta.chem.scand.13-1011.
- Diczfalusy, E; Fernö, O; Fex, H; Högberg, B; Linderot, T; Rosenberg, Th (1953). "Synthetic high molecular weight enzyme inhibitors. I. Polymeric phosphates of phloretin and related compounds" (PDF). Acta Chem Scand. 7 (6): 921–7. doi:10.3891/acta.chem.scand.07-0913.
- Picinelli, A; Dapena, E; Mangas, JJ (1995). "Polyphenolic pattern in apple tree leaves in relation to scab resistance. A preliminary study". Journal of Agricultural and Food Chemistry. 43 (8): 2273–78. doi:10.1021/jf00056a057.
- Svensk Farmaceutisk Tidskrift. Sveriges Apotekareförbunds. 1973. p. 728.
- http://www.micromedexsolutions.com
- Sweetman, Sean C., ed. (2009). "Sex hormones and their modulators". Martindale: The Complete Drug Reference (36th ed.). London: Pharmaceutical Press. p. 2082. ISBN 978-0-85369-840-1.
- Hartmann BW, Laml T, Kirchengast S, Albrecht AE, Huber JC (1998). "Hormonal breast augmentation: prognostic relevance of insulin-like growth factor-I". Gynecol. Endocrinol. 12 (2): 123–7. doi:10.3109/09513599809024960. PMID 9610425.
- Joseph William Goldzieher; Kenneth Fotherby (1994). Pharmacology of the contraceptive steroids. Raven Press. p. 154. ISBN 978-0-7817-0097-9.
- Zañartu J, Rice-Wray E, Goldzieher JW (October 1966). "Fertility control with long-acting injectable steroids. A preliminary report". Obstet Gynecol. 28 (4): 513–5. doi:10.1097/00006250-196610000-00011 (inactive 2021-01-20). PMID 5925038.CS1 maint: DOI inactive as of January 2021 (link)
- Harry Beckman (1967). The Year Book of Drug Therapy. Year Book Publishers.
Further reading
- Gunnarsson PO, Norlén BJ (1988). "Clinical pharmacology of polyestradiol phosphate". Prostate. 13 (4): 299–304. doi:10.1002/pros.2990130405. PMID 3217277. S2CID 33063805.
- von Schoultz B, Carlström K, Collste L, Eriksson A, Henriksson P, Pousette A, Stege R (1989). "Estrogen therapy and liver function--metabolic effects of oral and parenteral administration". Prostate. 14 (4): 389–95. doi:10.1002/pros.2990140410. PMID 2664738. S2CID 21510744.
- Henriksson P (1991). "Estrogen in patients with prostatic cancer. An assessment of the risks and benefits". Drug Saf. 6 (1): 47–53. doi:10.2165/00002018-199106010-00005. PMID 2029353. S2CID 39861824.
- Stege R, Sander S (March 1993). "Endokrin behandling av prostatacancer. En renessanse for parenteralt østrogen" [Endocrine treatment of prostatic cancer. A renaissance for parenteral estrogen]. Tidsskr. Nor. Laegeforen. (in Norwegian). 113 (7): 833–5. PMID 8480286.
- Stege R, Carlström K, Hedlund PO, Pousette A, von Schoultz B, Henriksson P (September 1995). "Intramuskuläres Depotöstrogen (Estradurin) in der Behandlung von Patienten mit Prostatakarzinom. Historische Aspekte, Wirkungsmechanismus, Resultate und aktueller klinischer Stand" [Intramuscular depot estrogens (Estradurin) in treatment of patients with prostate carcinoma. Historical aspects, mechanism of action, results and current clinical status]. Urologe A (in German). 34 (5): 398–403. ISSN 0340-2592. PMID 7483157.
- Smith PH, Robinson MR (September 1995). "Renaissance der Ostrogentherapie beim fortgeschrittenen Prostatakarzinom" [Renaissance of estrogen therapy in advanced prostate carcinoma?]. Urologe A (in German). 34 (5): 393–7. PMID 7483156.
- Cox RL, Crawford ED (December 1995). "Estrogens in the treatment of prostate cancer". J. Urol. 154 (6): 1991–8. doi:10.1016/S0022-5347(01)66670-9. PMID 7500443.
- Mikkola A, Ruutu M, Aro J, Rannikko S, Salo J (1999). "The role of parenteral polyestradiol phosphate in the treatment of advanced prostatic cancer on the threshold of the new millennium". Ann Chir Gynaecol. 88 (1): 18–21. ISSN 0355-9521. PMID 10230677.
- Oh WK (September 2002). "The evolving role of estrogen therapy in prostate cancer". Clin Prostate Cancer. 1 (2): 81–9. doi:10.3816/CGC.2002.n.009. PMID 15046698.
- Ockrim J, Lalani EN, Abel P (October 2006). "Therapy Insight: parenteral estrogen treatment for prostate cancer--a new dawn for an old therapy". Nat Clin Pract Oncol. 3 (10): 552–63. doi:10.1038/ncponc0602. PMID 17019433. S2CID 6847203.
- Lycette JL, Bland LB, Garzotto M, Beer TM (December 2006). "Parenteral estrogens for prostate cancer: can a new route of administration overcome old toxicities?". Clin Genitourin Cancer. 5 (3): 198–205. doi:10.3816/CGC.2006.n.037. PMID 17239273.
- Norman G, Dean ME, Langley RE, Hodges ZC, Ritchie G, Parmar MK, Sydes MR, Abel P, Eastwood AJ (February 2008). "Parenteral oestrogen in the treatment of prostate cancer: a systematic review". Br. J. Cancer. 98 (4): 697–707. doi:10.1038/sj.bjc.6604230. PMC 2259178. PMID 18268497.
- Ockrim J, Abel PD (2009). "Androgen deprivation therapy for prostate cancer – the potential of parenteral estrogen". Central European Journal of Urology. 62 (3): 132–140. doi:10.5173/ceju.2009.03.art1.
- Wibowo E, Schellhammer P, Wassersug RJ (January 2011). "Role of estrogen in normal male function: clinical implications for patients with prostate cancer on androgen deprivation therapy". J. Urol. 185 (1): 17–23. doi:10.1016/j.juro.2010.08.094. PMID 21074215.
- Wibowo E, Wassersug RJ (September 2013). "The effect of estrogen on the sexual interest of castrated males: Implications to prostate cancer patients on androgen-deprivation therapy". Crit. Rev. Oncol. Hematol. 87 (3): 224–38. doi:10.1016/j.critrevonc.2013.01.006. PMID 23484454.
- Phillips I, Shah SI, Duong T, Abel P, Langley RE (2014). "Androgen Deprivation Therapy and the Re-emergence of Parenteral Estrogen in Prostate Cancer". Oncol Hematol Rev. 10 (1): 42–47. doi:10.17925/OHR.2014.10.1.42. PMC 4052190. PMID 24932461.
- Ali Shah SI (2015). "Emerging potential of parenteral estrogen as androgen deprivation therapy for prostate cancer". South Asian J Cancer. 4 (2): 95–7. doi:10.4103/2278-330X.155699. PMC 4418092. PMID 25992351.
- Russell N, Cheung A, Grossmann M (August 2017). "Estradiol for the mitigation of adverse effects of androgen deprivation therapy". Endocr. Relat. Cancer. 24 (8): R297–R313. doi:10.1530/ERC-17-0153. PMID 28667081.