Presynaptic inhibition
Presynaptic inhibition is an inhibitory input to a neuron to make it less likely to fire an action potential and communicate with downstream neurons. Inhibition can be provided both at the postsynapse (IPSP) and the presynapse. Presynaptic inhibition occurs when an inhibitory neurotransmitter, like GABA, acts on GABA receptors on the axon terminal. Presynaptic inhibition is ubiquitous among sensory neurons.[1]
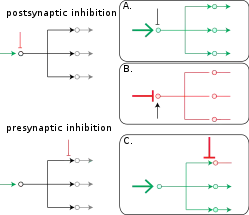
Function of presynaptic inhibition
Somatosensory neurons constantly provide information about the body's current state (e.g. temperature, pain, pressure, position, etc.); this constant influx of information is subject to modulation to enhance or diminish stimuli (see also: gate control theory and gain control-biological). Because there are unlimited stimuli at any given point to feel, it is imperative that these signals are appropriately filtered and compressed. To diminish certain stimuli, primary afferents receive inhibitory input (likely from GABA, but could also be glycine[2]) to reduce their synaptic output. Impaired presynaptic inhibition has been implicated in many neurological disorders, such as chronic pain, epilepsy, autism, and fragile-X syndrome.[3][4][5][6][7]
Mechanisms of presynaptic inhibition
The biophysical mechanism of presynaptic inhibition remains controversial. The presynaptic terminal has a distinct ionic composition that is high in chloride concentration which is largely due to cation-chloride cotransporters.[8] Typically when GABA receptors are activated, it causes a chloride influx, which hyperpolarizes the cell. However, due to the high concentration of chloride at the presynaptic terminal and its altered reversal potential, GABA receptor activation actually causes a chloride efflux, and a resulting depolarization. This phenomenon is called primary afferent depolarization (PAD). Despite the depolarized potential, this still results in a reduction of neurotransmitter release and thus is still inhibition. There are three hypotheses which propose mechanisms behind this paradox:[9][10][11][12][13][14][15][16][17]
- The depolarized membrane causes inactivation of voltage-gated sodium channels on the terminals and therefore the action potential is prevented from propagating
- Open GABA receptor channels act as a shunt, whereby current flows out of instead of concluding at the terminals
- The depolarized membrane causes inactivation of voltage-gated calcium channels, preventing calcium influx at the synapse (which is imperative for neurotransmission).
History of the discovery of presynaptic inhibition
1933: Grasser & Graham observed depolarization that originated in the sensory axon terminals[18]
1938: Baron & Matthews observed depolarization that originated in sensory axon terminals and the ventral root[19]
1957: Frank & Fuortes coined the term "presynaptic inhibition" [20]
1961: Eccles, Eccles, & Magni determined that the Dorsal Root Potential (DRP) originated from depolarization in sensory axon terminals [21]
References
- McGann JP (2013). "Presynaptic Inhibition of Olfactory Sensory Neurons: New Mechanisms and Potential Functions". Chem Senses. 38 (6): 459-574. doi:10.1093/chemse/bjt018. PMC 3685425. PMID 23761680.
- Geiman EJ, Zheng W, Fritschy JM, Alvarez FJ (2002). "Glycine and GABAA receptor subunits on Renshaw cells: Relationship with presynaptic neurotransmitters and postsynaptic gephyrin clusters". JCN. 444 (3): 275–289. doi:10.1002/cne.10148.
- Deidda G, Bozarth IF, Cancedda L (2014). "Modulation of GABAergic transmission in development and neurodevelopmental disorders: investigating physiology and pathology to gain therapeutic perspectives". Frontiers in Cellular Neuroscience. 8: 119. doi:10.3389/fncel.2014.00119. PMC 4033255. PMID 24904277.
- Zeilhofer HU, Wildner H, Yévenes GE (2012). "Fast synaptic inhibition in spinal sensory processing and pain control". Physiology Reviews. 92 (1): 193–235. doi:10.1152/physrev.00043.2010. PMC 3590010. PMID 22298656.
- Lee E, Lee J, Kim E (2017). "Excitation/Inhibition Imbalance in Animal Models of Autism Spectrum Disorders". Biol. Psychiatry. 81 (10): 838-847. doi:10.1016/j.biopsych.2016.05.011.
- D’Hulst C, Kooy HF (2007). "The GABAA receptor: a novel target for treatment of fragile X?". Trends Neurosci. 30 (8): 425-431. doi:10.1016/j.tins.2007.06.003. PMID 17590448.
- Benarroch EE (2007). "GABAA receptor heterogeneity, function, and implications for epilepsy". Neurology. 68 (8): 612-614. doi:10.1212/01.wnl.0000255669.83468.dd. PMID 17310035.
- Kahle KT, Staley KJ, Nahed BV, Gamba G, Hebert SC, Lifton RP, Mount DB (2008). "Roles of cation-chloride cotransporters in neurological disease". Nat Clin Pract Neurol. 4 (9): 490–503. doi:10.1038/ncpneuro0883. PMID 18769373.
- Guo D, Hu J (2014). "Spinal Presynaptic Inhibition in Pain Control". Neuroscience. 283: 95–106. doi:10.1016/j.neuroscience.2014.09.032.
- Rudomin P, Schmidt R (1999). "Presynaptic inhibition in the vertebrate spinal cord revisited". Exp Brain Res. 129 (1): 1–37. doi:10.1007/s002210050933.
- Price TJ, Cervero F, Gold MS, Hammond DL, Prescott SA (2009). "Chloride regulation in the pain pathway". Brain Res Rev. 60 (1): 149–170. doi:10.1016/j.brainresrev.2008.12.015. PMC 2903433. PMID 19167425.
- Cattaert D, El Manira A (1999). "Shunting versus inactivation: analysis of presynaptic inhibitory mechanisms in primary afferents of the crayfish". Neuroscience. 19 (14): 6079–6089. PMC 6783106. PMID 10407044.
- Willis WD (1999). "Dorsal root potentials and dorsal root reflexes: a double-edged sword". Exp Brain Res. 124 (4): 395–421. doi:10.1007/s002210050637.
- Willis WD (2006). "John Eccles' studies of spinal cord presynaptic inhibition". Prog Neurobiol. 78 (7–8): 189–214. doi:10.1016/j.pneurobio.2006.02.007. PMID 16650518.
- Cattaert D, Liberat F, El Manira AA (2001). "Presynaptic inhibition and antidromic spikes in primary afferents of the crayfish: a computational and experimental analysis". Neuroscience. 21 (3): 1007–1021. PMC 6762302. PMID 11157086.
- Panek I, French AS, Seyfarth EA, Sekizawa S, Torkkeli PH (2002). "Peripheral GABAergic inhibition of spider mechanosensory afferents". European Journal of Neuroscience. 16 (1): 96–104. doi:10.1046/j.1460-9568.2002.02065.x. PMID 12153534.
- French AS, Panek I, Torkkeli PH (2006). "Shunting versus inactivation: simulation of GABAergic inhibition in spider mechanoreceptors suggests that either is sufficient". Neuroscience Research. 55 (2): 189–196. doi:10.1016/j.neures.2006.03.002. PMID 16616790.
- Gasser & Graham (1933). "Potentials produced in the spinal cord by stimulation of dorsal roots". American Journal of Physiology. 103: 303–320.
- Barron & Matthews (1938). "The Interpretation of Potential Changes in the Spinal Cord". Journal of Physiology. 92: 276–321.
- Frank & Fuortes (1957). "Presynaptic and Postsynaptic inhibition of monsynaptic reflexes". Federation Proceedings. 16: 39–40.
- Eccles, Eccles, & Magni (1961). "Central inhibitory action attributable to presynaptic depolarization produced by muscle afferent volleys". Journal of Physiology (London). 159: 147–166.CS1 maint: multiple names: authors list (link)