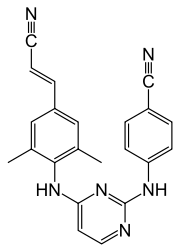
Rilpivirine
Rilpivirine, sold under the brand name Edurant among others, is a medication, developed by Tibotec, used for the treatment of HIV/AIDS.[5][6] It is a second-generation non-nucleoside reverse transcriptase inhibitor (NNRTI) with higher potency, longer half-life and reduced side-effect profile compared with older NNRTIs, such as efavirenz.[7][8]
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Edurant, Rekambys |
| Other names | TMC278 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a611037 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth, intramuscular |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Elimination half-life | 38 hours |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| DrugBank |
|
| ChemSpider | |
| UNII |
|
| KEGG | |
| ChEBI |
|
| ChEMBL |
|
| NIAID ChemDB | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.224.394 |
| Chemical and physical data | |
| Formula | C22H18N6 |
| Molar mass | 366.428 g·mol−1 |
| |
| | |
Rilpivirine entered phase III clinical trials in April 2008,[9][10] and was approved for use in the United States in May 2011, under the brand name Edurant.[11][12] Edurant is approved for treatment-naive patients with a viral load of 100,000 copies/mL or less at therapy initiation. It is contraindicated for use with proton pump inhibitors due to the increased gastric pH causing decreased rilpivirine plasma concentrations, potentially resulting in loss of virologic response and possible resistance. A fixed-dose medication combining rilpivirine with emtricitabine and tenofovir disoproxil (TDF), was approved by the U.S. Food and Drug Administration (FDA) in August 2011, under the brand name Complera,[13] and was approved for use in the European Union with the brand name Eviplera in November 2011.[14] A fixed-dose medication also combining rilpivirine with emtricitabine and tenofovir alafenamide (TAF) was approved for use in the US in March 2016, with the brand name Odefsey.[15]
Like etravirine, a second-generation NNRTI approved in 2008, rilpivirine is a diarylpyrimidine (DAPY). Rilpivirine in combination with emtricitabine and tenofovir has been shown to have higher rates of virologic failure than emtricitabine/tenofovir/efavirenz in people with baseline HIV viral loads greater than 100,000 copies/mm3.
On 15 October 2020, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) adopted a positive opinion, recommending the granting of a marketing authorization for the medicinal product Rekambys, intended for the treatment of human immunodeficiency virus type-1(HIV-1) infection in combination with cabotegravir injection.[16] Rekambys was approved for medical use in the European Union in December 2020.[4]
The EMA has recommended the granting of marketing authorizations for two new antiretroviral (ARV) medicines, Rekambys (rilpivirine) and Vocabria injection (cabotegravir), to be used together for the treatment of people with human immunodeficiency virus type 1 (HIV-1) infection.[17] The two medicines are the first antiretrovirals that come in a long-acting injectable formulation.[17] This means that instead of daily pills, people receive intramuscular injections monthly or every two months.[17]
The combination of Rekambys and Vocabria injection is intended for maintenance treatment of adults who have undetectable HIV levels in the blood (viral load less than 50 copies/ml) with their current antiretroviral treatment, and when the virus has not developed resistance to certain class of anti-HIV medicines called non-nucleoside reverse transcriptase inhibitors (NNRTIs) and integrase strand transfer inhibitors (INIs).[4][17]
In January 2021, the U.S. Food and Drug Administration (FDA) approved cabotegravir/rilpivirine (Cabenuva) injectable formulation as a complete regimen for the treatment of human immunodeficiency virus type 1 (HIV-1) infection in adults to replace a current antiretroviral regimen in those who are virologically suppressed on a stable antiretroviral regimen with no history of treatment failure and with no known or suspected resistance to either cabotegravir or rilpivirine.[18][19] This is the first FDA-approved injectable, complete regimen for HIV-infected adults that is administered once a month.[18][19] The label for rilpivirine tablets was revised to reflect the oral lead-in recommendations for use with cabotegravir.[19]
References
- "Edurant 25 mg tablets - Summary of Product Characteristics (SmPC)". (emc). 21 January 2020. Retrieved 4 January 2021.
- "Edurant- rilpivirine hydrochloride tablet, film coated". DailyMed. Retrieved 4 January 2021.
- "Edurant EPAR". European Medicines Agency (EMA). Retrieved 4 January 2021.
- "Rekambys EPAR". European Medicines Agency (EMA). 13 October 2020. Retrieved 4 January 2021.
- "TMC278 — A new NNRTI". Tibotec. Archived from the original on 2008-12-20. Retrieved 2010-03-07.
- Stellbrink HJ (2007). "Antiviral drugs in the treatment of AIDS: what is in the pipeline?". Eur. J. Med. Res. 12 (9): 483–95. PMID 17933730.
- Goebel F, Yakovlev A, Pozniak AL, Vinogradova E, Boogaerts G, Hoetelmans R, de Béthune MP, Peeters M, Woodfall B (2006). "Short-term antiviral activity of TMC278—a novel NNRTI—in treatment-naive HIV-1-infected subjects". AIDS. 20 (13): 1721–6. doi:10.1097/01.aids.0000242818.65215.bd. PMID 16931936. S2CID 26078073.
- Pozniak A, Morales-Ramirez J, Mohap L, et al. "48-Week Primary Analysis of Trial TMC278-C204: TMC278 Demonstrates Potent and Sustained Efficacy in ART-naïve Patients. Oral abstract 144LB". 14th Conference on Retroviruses and Opportunistic Infections. Archived from the original on October 19, 2007.
- "A Clinical Trial in Treatment naïve HIV-1 Patients Comparing TMC278 to Efavirenz in Combination With Tenofovir + Emtricitabine". ClinicalTrials.gov. National Institutes of Health. October 25, 2012. Retrieved January 1, 2014.
- "A Clinical Trial in Treatment naïve HIV-Subjects Patients Comparing TMC278 to Efavirenz in Combination With 2 Nucleoside/Nucleotide Reverse Transcriptase Inhibitors". ClinicalTrials.gov. National Institutes of Health. May 14, 2012. Retrieved January 1, 2014.
- "Drug Approval Package: Edurant (rilpivirine) NDA #202022#". U.S. Food and Drug Administration (FDA). 20 August 2013. Retrieved 23 January 2021. Lay summary (PDF).
- "FDA approves new HIV treatment" (Press release). U.S. Food and Drug Administration (FDA). Retrieved 2011-05-20.
- "Approval of Complera: emtricitabine/rilpivirine/tenofovir DF fixed dose combination". U.S. Food and Drug Administration (FDA). August 10, 2011.
- "Eviplera". Aidsmap. Retrieved September 1, 2014.
- "Odefsey (emtricitabine, rilpivirine, and tenofovir alafenamide) Tablets". U.S. Food and Drug Administration (FDA). 29 November 2016. Retrieved 23 January 2021.
- "Rekambys: Pending EC decision". European Medicines Agency (EMA). 16 October 2020. Retrieved 16 October 2020. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- "First long-acting injectable antiretroviral therapy for HIV recommended approval". European Medicines Agency (EMA) (Press release). 16 October 2020. Retrieved 16 October 2020. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- "FDA Approves First Extended-Release, Injectable Drug Regimen for Adults Living with HIV". U.S. Food and Drug Administration (FDA) (Press release). 21 January 2021. Retrieved 21 January 2021.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain. - "Cabenuva and Vocabria approved for HIV infection". U.S. Food and Drug Administration (FDA). 27 January 2021. Retrieved 27 January 2021.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
External links
- "Rilpivirine". Drug Information Portal. U.S. National Library of Medicine.
- "Rilpivirine hydrochloride". Drug Information Portal. U.S. National Library of Medicine.
