SMIM15
SMIM15(small integral membrane protein 15) is a protein in humans that is encoded by the SMIM15 gene.[5] It is a transmembrane protein that interacts with PBX4.[6] Deletions where SMIM15 is located have produced mental defects and physical deformities.[7][8] SMIM15 has been found to have ubiquitous but variable expression in many tissues throughout the body.[5]
| SMIM15 | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||||||||||||||||||
| Aliases | SMIM15, C5orf43, small integral membrane protein 15 | ||||||||||||||||||||||||
| External IDs | MGI: 1922866 HomoloGene: 90075 GeneCards: SMIM15 | ||||||||||||||||||||||||
| |||||||||||||||||||||||||
| |||||||||||||||||||||||||
| Orthologs | |||||||||||||||||||||||||
| Species | Human | Mouse | |||||||||||||||||||||||
| Entrez | |||||||||||||||||||||||||
| Ensembl | |||||||||||||||||||||||||
| UniProt | |||||||||||||||||||||||||
| RefSeq (mRNA) | |||||||||||||||||||||||||
| RefSeq (protein) | |||||||||||||||||||||||||
| Location (UCSC) | Chr 5: 61.16 – 61.16 Mb | Chr 13: 108.04 – 108.05 Mb | |||||||||||||||||||||||
| PubMed search | [3] | [4] | |||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||
| |||||||||||||||||||||||||
Gene
Small integral membrane protein 15 (SMIM15) is a protein in humans that is encoded by the SMIM15 gene.[5] It has also been known under the aliases C5orf43[5] and GC05M060454.[5] It is made up of 74 amino acids. It is located at 5q12.1.[5] SMIM15 has 4741 base pairs with three exons[5][9]
mRNA
SMIM15 has zero isoforms[5] The 5' UTR region spans 420 bases and the 3' UTR spans 2243 bases.[9]
| Exon | Number of Base Pairs | Start and End Locations |
| 1 | 252 | 61162217 – 61162468 |
| 2 | 140 | 61161088 – 61161227 |
| 3 | 2496 | 61157704 – 61160199 |
Protein
Primary sequence of SMIM15 is:[11] MFDIKAWAEY VVEWAAKDPY GFLTTVILAL TPLFLASAVL SWKLAKMIEA REKEQKKKQK. RQENIAKAKR LKKD
Molecular weight of SMIM15 has been found to be 8.6 kdal and it has a pI of 9.82.[12] There are no significant compositional features compositional features like charge clusters, hydrophobic segments, charge runs, patterns, multiplets or periodicities.[13]
Domains and motifs
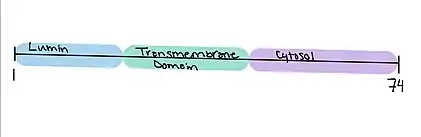
There is one transmembrane domain located from amino acids 20 – 42.[14][15]
The other domains include a luminal domain from amino acids 1 - 19 and cytosolic domain from amino acids 43 - 74.[14][15]
Secondary structure
The secondary structure for SMIM15 is largely alpha-helical with alpha helices making up 62.16% (46 amino acids) of the protein.[16] Random coil makes up 25.68% (19 amino acids) and extended strands make up 12.16% (9 amino acids) of the SMIM15 protein.[16]
Post-translational modifications
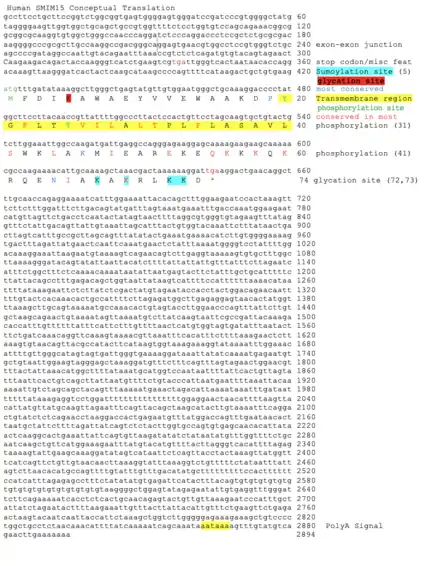
There are a number of post-translational modifications of the SMIM15 protein, which are shown in the Conceptual Translation of Human SMIM15 as shown in figure 1.
The predicted sites for sumoylation are at positions: 5, 67, 69, 72, 73.[17] It is known to affect protein stability, protect from degradation, cellular localization, protein-protein interactions and DNA binding.
The predicted sites for glycation are at positions: 5, 43, 58, 72, 73.[18] Glycation can lead to the creation of AGE (advanced glycation end products.[19] Glycation is a process in which proteins react with reducing sugar molecules, which will lead to impairment of the function and changes the characteristics of the protein.[20][21]
Finally, there are four predicted sites for phosphorylation of tyrosine on position 20, threonine on positions 25 and 31, and serine on position 41.[22] Phosphorylation will affect different cellular processes and thus regulating protein function.[23]
Subcellular localization
SMIM15 has a transmembrane domain found within amino acids 20-42. There are cleavage sites at the C-terminous and nuclear localization signals.[24]
Expression
SMIM15 has been found to have ubiquitous but variable expression in many different tissues throughout the body.[5] it has the highest level of expression within the prostate.[25] There are lower levels of expression within skeletal muscles compared to other tissues within the body.[26]
Epigenetic
SMIM15 has one CpG island within the promoter. SMIM15 has lower levels of H3K4Me1 but higher levels of H3K4Me3 and H3K27Ac across all of their cell lines[27]
Transcriptional
The Promoter region for SMIM15 is 1049 base pairs long.[10] and it is known as GXP_922465. There are 431 different transcription binding factor sites,[10] some of these binding factors include GATA1, TGIF, LMX1A, and NKX61[10]
Translational and mRNA stability
There are no known micro-RNA targets in the 3' UTR.[10] mRNA secondary structures exhibited a high number of predicted stem-loop structures. This could indicate high stability of the mRNA transcript, and some binding sites for regulatory mechanisms.
Function
The function of SMIM15 is currently not well understood.
Interacting Proteins
There is only one interacting protein currently identified.[28][29] This protein is PBX4 which is known for playing critical roles in embryonic development and cellular differentiation both as Hox cofactors and through Hox - independent pathways.[6] PBX4 is also a member of the pre-B cell leukemia transcription factor family.[6][30]
Clinical Significance
Deletion of 5q12.1 can lead to the development of mental retardation and ocular defects.[7] Another deletion in the 5q12.1 - 5q12.3 region lead to mental-motor retardation and dysmorphia.[8] In terms of diseases, Caries is a multifactorial disease and little is still known about the host genetic factors influencing susceptibility. The interval 5q12.1-5q13.3 as linked to low caries susceptibility in Filipino families.[31]
Homology
SMIM15 is conserved in both vertebrates and invertebrates. It is not found in insects or fungi. SMIM15 does not have any paralogs[5] and the farthest known relative of the Homo sapiens SMIM15 is found within Trichoplax sp.H2 with a date of divergence 747 MYA[32]
References
- GRCh38: Ensembl release 89: ENSG00000188725 - Ensembl, May 2017
- GRCm38: Ensembl release 89: ENSMUSG00000071180 - Ensembl, May 2017
- "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- "SMIM15 small integral membrane protein 15 [Homo sapiens (human)] - Gene - NCBI". www.ncbi.nlm.nih.gov. Retrieved 2020-05-03.
- "PBX4 PBX homeobox 4 [Homo sapiens (human)] - Gene - NCBI". www.ncbi.nlm.nih.gov. Retrieved 2020-05-03.
- Jaillard, Sylvie; Andrieux, Joris; Plessis, Ghislaine; Krepischi, Ana C. V.; Lucas, Josette; David, Véronique; Le Brun, Marine; Bertola, Debora R.; David, Albert; Belaud-Rotureau, Marc-Antoine; Mosser, Jean (April 2011). "5q12.1 deletion: delineation of a phenotype including mental retardation and ocular defects". American Journal of Medical Genetics. Part A. 155A (4): 725–731. doi:10.1002/ajmg.a.33758. ISSN 1552-4833. PMID 21594994.
- Cetin, Zafer; Yakut, Sezin; Clark, Ozden Altiok; Mihci, Ercan; Berker, Sibel; Luleci, Guven (2013-03-01). "A 5q12.1-5q12.3 microdeletion in a case with a balanced exceptional complex chromosomal rearrangement". Gene. 516 (1): 176–180. doi:10.1016/j.gene.2012.12.013. ISSN 1879-0038. PMID 23262338.
- "Human Gene SMIM15 (ENST00000339020.8) Description and Page Index". genome.ucsc.edu. Retrieved 2020-05-03.
- "Genomatix: Annotation & Analysis". www.genomatix.de. Retrieved 2020-05-03.
- "small integral membrane protein 15 [Homo sapiens] - Protein - NCBI". www.ncbi.nlm.nih.gov. Retrieved 2020-05-03.
- "ExPASy - Compute pI/Mw tool". web.expasy.org. Retrieved 2020-05-03.
- "SAPS < Sequence Statistics < EMBL-EBI". www.ebi.ac.uk. Retrieved 2020-05-03.
- elm.eu.org http://elm.eu.org/cgimodel.py?fun=smartResult&userId=QiKRz8j5Na&EXPECT_CUTOFF=100&r=1&bg=on#TRG_NLS_Bipartite_1:in. Retrieved 2020-05-03. Missing or empty
|title=(help) - "5E97AC2D00000D9E239DE439 expired". www.cbs.dtu.dk. Retrieved 2020-05-03.
- npsa-prabi.ibcp.fr https://npsa-prabi.ibcp.fr/cgi-bin/secpred_gor4.pl. Retrieved 2020-05-03. Missing or empty
|title=(help) - "GPS-SUMO: Prediction of SUMOylation Sites & SUMO-interaction Motifs". sumosp.biocuckoo.org. Retrieved 2020-05-03.
- "NetGlycate 1.0 Server". www.cbs.dtu.dk. Retrieved 2020-05-03.
- Kim, Chan-Sik; Park, Sok; Kim, Junghyun (2017-09-30). "The role of glycation in the pathogenesis of aging and its prevention through herbal products and physical exercise". Journal of Exercise Nutrition & Biochemistry. 21 (3): 55–61. doi:10.20463/jenb.2017.0027. ISSN 2233-6834. PMC 5643203. PMID 29036767.
- "Glycation", Wikipedia, 2020-03-25, retrieved 2020-05-03
- Johansen, Morten Bo; Kiemer, Lars; Brunak, Søren (September 2006). "Analysis and prediction of mammalian protein glycation". Glycobiology. 16 (9): 844–853. doi:10.1093/glycob/cwl009. ISSN 0959-6658. PMID 16762979.
- "5E9CF8E40000064EBE8DB075 expired". www.cbs.dtu.dk. Retrieved 2020-05-03.
- "Phosphorylation - US". www.thermofisher.com. Retrieved 2020-05-03.
- "ELM - Detail for TRG_NLS_Bipartite_1". elm.eu.org. Retrieved 2020-05-03.
- "GDS423 / 45795_at". www.ncbi.nlm.nih.gov. Retrieved 2020-05-03.
- "Tissue expression of SMIM15 - Summary - The Human Protein Atlas". www.proteinatlas.org. Retrieved 2020-05-03.
- "Human hg38 chr5:61,153,557-61,167,851 UCSC Genome Browser v397". genome.ucsc.edu. Retrieved 2020-05-03.
- "Home < IMEx". www.imexconsortium.org. Retrieved 2020-05-03.
- www.ebi.ac.uk https://www.ebi.ac.uk/intact/?conversationContext=1. Retrieved 2020-05-03. Missing or empty
|title=(help) - Laurent, Audrey; Bihan, Réjane; Omilli, Francis; Deschamps, Stéphane; Pellerin, Isabelle (2008). "PBX proteins: much more than Hox cofactors". The International Journal of Developmental Biology. 52 (1): 9–20. doi:10.1387/ijdb.072304al. ISSN 0214-6282. PMID 18033668.
- Shimizu, T.; Deeley, K.; Briseño-Ruiz, J.; Faraco, I. M.; Poletta, F. A.; Brancher, J. A.; Pecharki, G. D.; Küchler, E. C.; Tannure, P. N.; Lips, A.; Vieira, T. C. S. (2013). "Fine-mapping of 5q12.1-13.3 unveils new genetic contributors to caries". Caries Research. 47 (4): 273–283. doi:10.1159/000346278. ISSN 1421-976X. PMC 3737367. PMID 23363935.
- "TimeTree :: The Timescale of Life". www.timetree.org. Retrieved 2020-05-03.