SWI/SNF
In molecular biology, SWI/SNF (SWItch/Sucrose Non-Fermentable),[1][2] is a subfamiliy of ATP-dependent chromatin remodeling complexes, which is found in eukaryotes. In other words, it is a group of proteins that associate to remodel the way DNA is packaged. This complex is composed of several proteins – products of the SWI and SNF genes (SWI1, SWI2/SNF2, SWI3, SWI5, SWI6), as well as other polypeptides.[3] It possesses a DNA-stimulated ATPase activity that can destabilize histone-DNA interactions in reconstituted nucleosomes in an ATP-dependent manner, though the exact nature of this structural change is unknown. The SWI/SNF subfamily provides crucial nucleosome rearrangement, which is seen as ejection and/or sliding. The movement of nucleosomes provides easier access to the chromatin, allowing genes to be activated or repressed.[4]
| Snf2 ATPase bound to a nucleosome | |||||||||
|---|---|---|---|---|---|---|---|---|---|
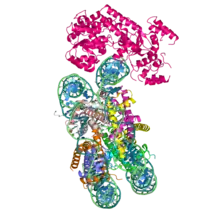 Cryo-EM reconstruction of S. cerevisiae Snf2 ATPase in complex with a nucleosome | |||||||||
| Identifiers | |||||||||
| Symbol | Snf2 | ||||||||
| Pfam | PF00176 | ||||||||
| InterPro | IPR000330 | ||||||||
| SMART | DEXDc | ||||||||
| SCOP2 | 5x0x / SCOPe / SUPFAM | ||||||||
| |||||||||
The human analogs of SWI/SNF are "BRG1- or BRM-associated factors", or BAF (SWI/SNF-A) and "Polybromo-associated BAF", which is also known as PBAF (SWI/SNF-B).[5] There are also Drosophila analogs of SWI/SNF, known as "Brahma Associated Protein", or BAP and "Polybromo-associated BAP", also known as PBAP.[6]
Mechanism of action
It has been found that the SWI/SNF complex (in yeast) is capable of altering the position of nucleosomes along DNA.[7] These alterations are classified in three different ways, and they are seen as the processes of sliding nucleosomes, ejecting nucleosomes, and ejecting only certain components of the nucleosome.[4] Due to the actions performed by the SWI/SNF subfamily, they are referred to as "access remodellers" and promote gene expression by exposing binding sites so that transcription factors can bind more easily.[4] Two mechanisms for nucleosome remodeling by SWI/SNF have been proposed.[8] The first model contends that a unidirectional diffusion of a twist defect within the nucleosomal DNA results in a corkscrew-like propagation of DNA over the octamer surface that initiates at the DNA entry site of the nucleosome. The other is known as the "bulge" or "loop-recapture" mechanism and it involves the dissociation of DNA at the edge of the nucleosome with re-association of DNA inside the nucleosome, forming a DNA bulge on the octamer surface. The DNA loop would then propagate across the surface of the histone octamer in a wave-like manner, resulting in the re-positioning of DNA without changes in the total number of histone-DNA contacts.[9] A recent study[10] has provided strong evidence against the twist diffusion mechanism and has further strengthened the loop-recapture model.
Role as a tumor suppressor
The mammalian SWI/SNF (mSWI/SNF) complex functions as a tumor suppressor in many human malignant cancers.[11] Early studies identified that SWI/SNF subunits were frequently absent in cancer cell lines.[12] SWI/SNF was first identified in 1998 as a tumor suppressor in rhabdoid tumors, a rare pediatric malignant cancer.[13] Other instances of SWI/SNF acting as a tumor suppressor comes from the heterozygous deletion of BAF47[14] or alteration of BAF47.[15] These instances result in cases of chronic and acute CML and in rarer cases, Hodgkin's lymphoma, respectively. To prove that BAF47, also known as SMARCB1, acts as a tumor suppressor, experiments resulting in the formation of rhabdoid tumors in mice were conducted via total knockout of BAF47.[16] As DNA sequencing costs diminished, many tumors were sequenced for the first time around 2010. Several of these studies revealed SWI/SNF to be a tumor suppressor in a number of diverse malignancies.[17][18][19][20] Several studies revealed that subunits of the mammalian complex, including ARID1A,[21] PBRM1,[20] SMARCB1,[22] SMARCA4,[23] and ARID2,[18] are frequently mutated in human cancers. It has been noted that total loss of BAF47 is extremely rare and instead, most cases of tumors that resulted from SWI/SNF subunits come from BRG1 deletion, BRM deletion, or total loss of both subunits.[24] Further analysis concluded that total loss of both subunits was present in about 10% of tumor cell lines after 100 cell lines were looked at.[25] A meta-analysis of many sequencing studies demonstrated SWI/SNF to be mutated in approximately 20% of human malignancies.[26]
Structure of the SWI/SNF complex
Electron microscopy studies of SWI/SNF and RSC (SWI/SNF-B) reveal large, lobed 1.1-1.3 MDa structures.[27][28][29][30] These structures resemble RecA and cover both sides of a conserved section of the ATPase domain. The domain also contains a separate domain, HSA, that is capable of binding actin, and resides on the N-terminus.[4] The bromo domain present is responsible for recognizing and binding lysines that have been acetylated.[6] No atomic-resolution structures of the entire SWI/SNF complex have been obtained to date, due to the protein complex being highly dynamic and composed of many subunits. However, domains and several individual subunits from yeast and mammals have been described. In particular, the cryo-EM structure of the ATPase Snf2 in complex with a nucleosome shows that nucleosomal DNA is locally deformed at the site of binding.[31] A model of the mammalian ATPase SMARCA4 shows similar features,[23] based on the high degree of sequence homology with yeast Snf2. The interface between two subunits, BAF155 (SMARCC1) and BAF47 (SMARCB1) was also resolved, providing important insights into the mechanisms of the SWI/SNF complex assembly pathway.[32]
SWIB/MDM2 protein domain
The protein domain, SWIB/MDM2, short for SWI/SNF complex B/MDM2 is an important domain. This protein domain has been found in both SWI/SNF complex B and in the negative regulator of the p53 tumor suppressor MDM2. It has been shown that MDM2 is homologous to the SWIB complex.[33]
Function
The primary function of the SWIB protein domain is to aid gene expression. In yeast, this protein domain expresses certain genes, in particular BADH2, GAL1, GAL4, and SUC2. It works by increasing transcription. It has ATPase activity, meaning it breaks down ATP, the basic unit of energy currency. This destabilizes the interaction between DNA and histones. The destabilization that occurs disrupts chromatin and opens up the transcription-binding domains. Transcription factors can then bind to this site, leading to an increase in transcription.[34]
Protein interaction
The interactions between the proteins of the SWI/SNF complex and the chromatin allows binding of transcription factors, therefore causing an increase in transcription.[34]
Structure
This protein domain is known to contain one short alpha helix.
Family members
Below is a list of yeast SWI/SNF family members with human and Drosophila[35] orthologs:[36]
| Yeast | Human | Drosophila | Function |
|---|---|---|---|
| SWI1 | ARID1A, ARID1B | OSA | Contains LXXLL nuclear receptor binding motifs |
| SWI2/SNF2 | SMARCA2, SMARCA4 | BRM | ATP dependent chromatin remodeling |
| SWI3 | SMARCC1, SMARCC2 | Moira/BAP155 | Similar sequence; function unknown |
| SWP73/SNF12 | SMARCD1, SMARCD2, SMARCD3 | BAP60 | Similar sequence; function unknown |
| SWP61/ARP7 | ACTL6A, ACTL6B | Actin-like protein | |
| SNF5 | SMARCB1 | SNR1 | ATP dependent chromatin remodeling |
History
The SWI/SNF complex was first discovered in the yeast, Saccharomyces cerevisiae. It was named after initially screening for mutations that would affect the pathways for both yeast mating types switching (SWI) and sucrose non-fermenting (SNF).[34][6]
References
- Neigeborn L, Carlson M (December 1984). "Genes affecting the regulation of SUC2 gene expression by glucose repression in Saccharomyces cerevisiae". Genetics. 108 (4): 845–58. PMC 1224269. PMID 6392017.
- Stern M, Jensen R, Herskowitz I (October 1984). "Five SWI genes are required for expression of the HO gene in yeast". Journal of Molecular Biology. 178 (4): 853–68. doi:10.1016/0022-2836(84)90315-2. PMID 6436497.
- Pazin MJ, Kadonaga JT (March 1997). "SWI2/SNF2 and related proteins: ATP-driven motors that disrupt protein-DNA interactions?". Cell. 88 (6): 737–40. doi:10.1016/S0092-8674(00)81918-2. PMID 9118215.
- Clapier CR, Iwasa J, Cairns BR, Peterson CL (July 2017). "Mechanisms of action and regulation of ATP-dependent chromatin-remodelling complexes". Nature Reviews. Molecular Cell Biology. 18 (7): 407–422. doi:10.1038/nrm.2017.26. PMID 28512350.
- Nie Z, Yan Z, Chen EH, Sechi S, Ling C, Zhou S, et al. (April 2003). "Novel SWI/SNF chromatin-remodeling complexes contain a mixed-lineage leukemia chromosomal translocation partner". Molecular and Cellular Biology. 23 (8): 2942–52. doi:10.1128/MCB.23.8.2942-2952.2003. PMC 152562. PMID 12665591.
- Tang L, Nogales E, Ciferri C (June 2010). "Structure and function of SWI/SNF chromatin remodeling complexes and mechanistic implications for transcription". Progress in Biophysics and Molecular Biology. 102 (2–3): 122–8. doi:10.1016/j.pbiomolbio.2010.05.001. PMC 2924208. PMID 20493208.
- Whitehouse I, Flaus A, Cairns BR, White MF, Workman JL, Owen-Hughes T (August 1999). "Nucleosome mobilization catalysed by the yeast SWI/SNF complex". Nature. 400 (6746): 784–7. Bibcode:1999Natur.400..784W. doi:10.1038/23506. PMID 10466730.
- van Holde K, Yager T (June 2003). "Models for chromatin remodeling: a critical comparison". Biochemistry and Cell Biology. 81 (3): 169–72. doi:10.1139/o03-038. PMID 12897850.
- Flaus A, Owen-Hughes T (April 2003). "Mechanisms for nucleosome mobilization". Biopolymers. 68 (4): 563–78. doi:10.1002/bip.10323. PMID 12666181.
- Zofall M, Persinger J, Kassabov SR, Bartholomew B (April 2006). "Chromatin remodeling by ISW2 and SWI/SNF requires DNA translocation inside the nucleosome". Nature Structural & Molecular Biology. 13 (4): 339–46. doi:10.1038/nsmb1071. PMID 16518397.
- Hodges C, Kirkland JG, Crabtree GR (August 2016). "The Many Roles of BAF (mSWI/SNF) and PBAF Complexes in Cancer". Cold Spring Harbor Perspectives in Medicine. 6 (8): a026930. doi:10.1101/cshperspect.a026930. PMC 4968166. PMID 27413115.
- Dunaief JL, Strober BE, Guha S, Khavari PA, Alin K, Luban J, et al. (October 1994). "The retinoblastoma protein and BRG1 form a complex and cooperate to induce cell cycle arrest". Cell. 79 (1): 119–30. doi:10.1016/0092-8674(94)90405-7. PMID 7923370.
- Versteege I, Sévenet N, Lange J, Rousseau-Merck MF, Ambros P, Handgretinger R, et al. (July 1998). "Truncating mutations of hSNF5/INI1 in aggressive paediatric cancer". Nature. 394 (6689): 203–6. Bibcode:1998Natur.394..203V. doi:10.1038/28212. PMID 9671307.
- Melo JV, Gordon DE, Cross NC, Goldman JM (January 1993). "The ABL-BCR fusion gene is expressed in chronic myeloid leukemia". Blood. 81 (1): 158–65. doi:10.1182/blood.v81.1.158.bloodjournal811158. PMID 8417787.
- Yuge M, Nagai H, Uchida T, Murate T, Hayashi Y, Hotta T, et al. (October 2000). "HSNF5/INI1 gene mutations in lymphoid malignancy". Cancer Genetics and Cytogenetics. 122 (1): 37–42. doi:10.1016/s0165-4608(00)00274-0. PMID 11104031.
- Reisman D, Glaros S, Thompson EA (April 2009). "The SWI/SNF complex and cancer". Oncogene. 28 (14): 1653–68. doi:10.1038/onc.2009.4. PMID 19234488.
- Wiegand KC, Shah SP, Al-Agha OM, Zhao Y, Tse K, Zeng T, et al. (October 2010). "ARID1A mutations in endometriosis-associated ovarian carcinomas". The New England Journal of Medicine. 363 (16): 1532–43. doi:10.1056/NEJMoa1008433. PMC 2976679. PMID 20942669.
- Li M, Zhao H, Zhang X, Wood LD, Anders RA, Choti MA, et al. (August 2011). "Inactivating mutations of the chromatin remodeling gene ARID2 in hepatocellular carcinoma". Nature Genetics. 43 (9): 828–9. doi:10.1038/ng.903. PMC 3163746. PMID 21822264.
- Shain AH, Giacomini CP, Matsukuma K, Karikari CA, Bashyam MD, Hidalgo M, et al. (January 2012). "Convergent structural alterations define SWItch/Sucrose NonFermentable (SWI/SNF) chromatin remodeler as a central tumor suppressive complex in pancreatic cancer". Proceedings of the National Academy of Sciences of the United States of America. 109 (5): E252-9. doi:10.1073/pnas.1114817109. PMC 3277150. PMID 22233809.
- Varela I, Tarpey P, Raine K, Huang D, Ong CK, Stephens P, et al. (January 2011). "Exome sequencing identifies frequent mutation of the SWI/SNF complex gene PBRM1 in renal carcinoma". Nature. 469 (7331): 539–42. Bibcode:2011Natur.469..539V. doi:10.1038/nature09639. PMC 3030920. PMID 21248752.
- Mathur R, Alver BH, San Roman AK, Wilson BG, Wang X, Agoston AT, et al. (February 2017). "ARID1A loss impairs enhancer-mediated gene regulation and drives colon cancer in mice". Nature Genetics. 49 (2): 296–302. doi:10.1038/ng.3744. PMC 5285448. PMID 27941798.
- Isakoff MS, Sansam CG, Tamayo P, Subramanian A, Evans JA, Fillmore CM, et al. (December 2005). "Inactivation of the Snf5 tumor suppressor stimulates cell cycle progression and cooperates with p53 loss in oncogenic transformation". Proceedings of the National Academy of Sciences of the United States of America. 102 (49): 17745–50. Bibcode:2005PNAS..10217745I. doi:10.1073/pnas.0509014102. PMC 1308926. PMID 16301525.
- Hodges HC, Stanton BZ, Cermakova K, Chang CY, Miller EL, Kirkland JG, et al. (January 2018). "Dominant-negative SMARCA4 mutants alter the accessibility landscape of tissue-unrestricted enhancers". Nature Structural & Molecular Biology. 25 (1): 61–72. doi:10.1038/s41594-017-0007-3. PMC 5909405. PMID 29323272.
- Muchardt C, Yaniv M (May 2001). "When the SWI/SNF complex remodels...the cell cycle". Oncogene. 20 (24): 3067–75. doi:10.1038/sj.onc.1204331. PMID 11420722.
- Decristofaro MF, Betz BL, Rorie CJ, Reisman DN, Wang W, Weissman BE (January 2001). "Characterization of SWI/SNF protein expression in human breast cancer cell lines and other malignancies". Journal of Cellular Physiology. 186 (1): 136–45. doi:10.1002/1097-4652(200101)186:1<136::aid-jcp1010>3.0.co;2-4. PMID 11147808.
- Shain AH, Pollack JR (2013). "The spectrum of SWI/SNF mutations, ubiquitous in human cancers". PLOS One. 8 (1): e55119. Bibcode:2013PLoSO...855119S. doi:10.1371/journal.pone.0055119. PMC 3552954. PMID 23355908.
- Asturias FJ, Chung WH, Kornberg RD, Lorch Y (October 2002). "Structural analysis of the RSC chromatin-remodeling complex". Proceedings of the National Academy of Sciences of the United States of America. 99 (21): 13477–80. Bibcode:2002PNAS...9913477A. doi:10.1073/pnas.162504299. PMC 129698. PMID 12368485.
- Leschziner AE, Saha A, Wittmeyer J, Zhang Y, Bustamante C, Cairns BR, Nogales E (March 2007). "Conformational flexibility in the chromatin remodeler RSC observed by electron microscopy and the orthogonal tilt reconstruction method". Proceedings of the National Academy of Sciences of the United States of America. 104 (12): 4913–8. Bibcode:2007PNAS..104.4913L. doi:10.1073/pnas.0700706104. PMC 1820885. PMID 17360331.
- Smith CL, Horowitz-Scherer R, Flanagan JF, Woodcock CL, Peterson CL (February 2003). "Structural analysis of the yeast SWI/SNF chromatin remodeling complex". Nature Structural Biology. 10 (2): 141–5. doi:10.1038/nsb888. PMID 12524530.
- Chaban Y, Ezeokonkwo C, Chung WH, Zhang F, Kornberg RD, Maier-Davis B, Lorch Y, Asturias FJ (December 2008). "Structure of a RSC-nucleosome complex and insights into chromatin remodeling". Nature Structural & Molecular Biology. 15 (12): 1272–7. doi:10.1038/nsmb.1524. PMC 2659406. PMID 19029894.
- Liu X, Li M, Xia X, Li X, Chen Z (April 2017). "Mechanism of chromatin remodelling revealed by the Snf2-nucleosome structure". Nature. 544 (7651): 440–445. Bibcode:2017Natur.544..440L. doi:10.1038/nature22036. PMID 28424519.
- Yan L, Xie S, Du Y, Qian C (June 2017). "Structural Insights into BAF47 and BAF155 Complex Formation". Journal of Molecular Biology. 429 (11): 1650–1660. doi:10.1016/j.jmb.2017.04.008. PMID 28438634.
- Bennett-Lovsey R, Hart SE, Shirai H, Mizuguchi K (April 2002). "The SWIB and the MDM2 domains are homologous and share a common fold". Bioinformatics. 18 (4): 626–30. doi:10.1093/bioinformatics/18.4.626. PMID 12016060.
- Decristofaro MF, Betz BL, Rorie CJ, Reisman DN, Wang W, Weissman BE (January 2001). "Characterization of SWI/SNF protein expression in human breast cancer cell lines and other malignancies". Journal of Cellular Physiology. 186 (1): 136–45. doi:10.1002/1097-4652(200101)186:1<136::AID-JCP1010>3.0.CO;2-4. PMID 11147808.
- "Table 1 The different components in the yeast, Drosophila and mammalian SWI/SNF complex". ISSN 1476-5594. Cite journal requires
|journal=(help) - Collingwood TN, Urnov FD, Wolffe AP (December 1999). "Nuclear receptors: coactivators, corepressors and chromatin remodeling in the control of transcription". Journal of Molecular Endocrinology. 23 (3): 255–75. doi:10.1677/jme.0.0230255. PMID 10601972.