Sakurai reaction
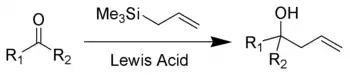
The Sakurai reaction (also known as the Hosomi–Sakurai reaction) is the chemical reaction of carbon electrophiles (such as a ketone shown here) with allylic silanes catalyzed by strong Lewis acids.[1][2][3][4][5][6] It is named after the chemists Akira Hosomi and Hideki Sakurai.
| Sakurai reaction | |
|---|---|
| Named after | Hideki Sakurai |
| Reaction type | Addition reaction |
| Identifiers | |
| Organic Chemistry Portal | hosomi-sakurai-reaction |
| RSC ontology ID | RXNO:0000443 |
Lewis acid activation is essential for complete reaction. Strong Lewis acids such as titanium tetrachloride, boron trifluoride, tin tetrachloride, and AlCl(Et)2 are all effective in promoting the Hosomi reaction. The reaction is a type of electrophilic allyl shift with formation of an intermediate beta-silyl carbocation. Driving force is the stabilization of said carbocation by the beta-silicon effect.
Various reactions
The Hosomi-Sakurai reaction can be performed on a number of functional groups. An electrophilic carbon, activated by a Lewis acid, is required. Below is a list of different functional groups that can be used in the Hosomi–Sakurai reaction.
Mechanism
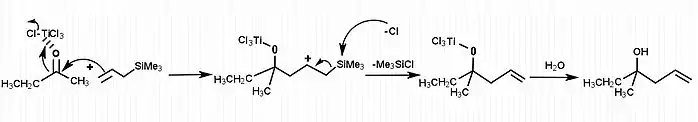
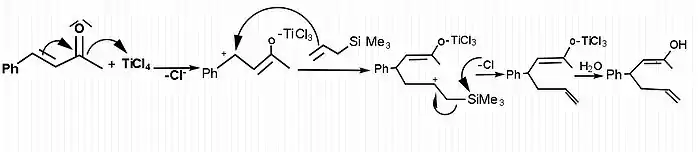
[7]The Hosomi-Sakurai reactions are allylation reactions which involve use of allyl silanes as allylmetal reagents. This section demonstrates examples of allylation of different ketone groups. In figure 1, allylation of a carbonyl ketone (compound containing a ketone group and two different functional groups) has been shown. In the given reaction, the electrophilic compound (carbon with a ketone group) is treated with titanium tetrachloride, a strong Lewis acid and allyltrimethylsilane. According to the general principle, the Lewis acid first activates the electrophilic carbon in presence of allyltrimethylsilane which then undergoes nucleophilic attack from electrons on the allylic silane.[8] The silicon plays the key role in stabilizing the carbocation of carbon at the β-position. Hosomi-Sakurai reaction is also applicable for other functional groups such as enones, where conjugate addition is usually seen. In figure 2, the Hosomi- Sakurai reaction has been shown using a cinnamoyl ketone. This reaction follows the same mechanism as the previous reaction shown here.
Beta-silicon effect stabilization
As displayed in the mechanism, the Hosomi–Sakurai reaction goes through a secondary carbocation intermediate. Secondary carbocations are inherently unstable, however the β-silicon effect from the silicon atom stabilizes the carbocation. Silicon is able to donate into an empty p-orbital, and the silicon orbital is shared between the two carbons. This stabilizes the positive charge over 3 orbitals. Another term for the β-silicon effect is silicon-hyperconjugation. This interaction is essential for the reaction to go to completion.
See also
References
- Hosomi, Akíra; Sakurai, Hideki (1 April 1976). "Syntheses of γ,δ-unsaturated alcohols from allylsilanes and carbonyl compounds in the presence of titanium tetrachloride". Tetrahedron Letters. 17 (16): 1295–1298. doi:10.1016/S0040-4039(00)78044-0. ISSN 0040-4039.
- Hosomi, Akira; Endo, Masahiko; Sakurai, Hideki (5 September 1976). "Allylsilanes as synthetic intermediates. ii. syntheses of homoallyl ethers from allylsilanes and acetals promoted by titanium tetrachloride". Chemistry Letters. 5 (9): 941–942. doi:10.1246/cl.1976.941. ISSN 0366-7022.
- Hosomi, Akira; Sakurai, Hideki (1 March 1977). "Chemistry of organosilicon compounds. 99. Conjugate addition of allylsilanes to .alpha.,.beta.-enones. A New method of stereoselective introduction of the angular allyl group in fused cyclic .alpha.,.beta.-enones". Journal of the American Chemical Society. 99 (5): 1673–1675. doi:10.1021/ja00447a080. ISSN 0002-7863.
- Hosomi, Akira (1 May 1988). "Characteristics in the reactions of allylsilanes and their applications to versatile synthetic equivalents". Accounts of Chemical Research. 21 (5): 200–206. doi:10.1021/ar00149a004. ISSN 0001-4842.
- Fleming, Ian; Dunoguès, Jacques; Smithers, Roger (2004), "The Electrophilic Substitution of Allylsilanes and Vinylsilanes", Organic Reactions, American Cancer Society, pp. 57–575, doi:10.1002/0471264180.or037.02, ISBN 9780471264187
- Fleming, Ian (1 January 1991). "Allylsilanes, Allylstannanes and Related Systems". In Trost, Barry M.; Fleming, Ian (eds.). 2.2 - Allylsilanes, Allylstannanes and Related Systems. Comprehensive Organic Synthesis. Pergamon. pp. 563–593. doi:10.1016/b978-0-08-052349-1.00041-x. ISBN 9780080523491. Retrieved 1 September 2019.
- "Hosomi-Sakurai Reaction". www.organic-chemistry.org. Retrieved 1 September 2019.
- Yamasaki, Shingo; Fujii, Kunihiko; Wada, Reiko; Kanai, Motomu; Shibasaki, Masakatsu (1 June 2002). "A General Catalytic Allylation Using Allyltrimethoxysilane". Journal of the American Chemical Society. 124 (23): 6536–6537. doi:10.1021/ja0262582. ISSN 0002-7863. PMID 12047165.
External links
- Hosomi-Sakurai reaction @ www.organic-chemistry.org Link
- Akira Hosomi HP