Samidorphan
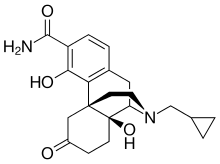
Samidorphan (INN, USAN) (developmental code names ALKS-33, RDC-0313), also known as 3-carboxamido-4-hydroxynaltrexone,[2] is an opioid antagonist that preferentially acts as an antagonist of the μ-opioid receptor (MOR). It is under development by Alkermes for the treatment of major depressive disorder and possibly other psychiatric conditions.[3]
 | |
| Clinical data | |
|---|---|
| Other names | ALKS-33, RDC-0313; 3-Carboxamido-4-hydroxynaltrexone |
| Routes of administration | Oral |
| Pharmacokinetic data | |
| Elimination half-life | 7–9 hours[1] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
| Formula | C21H26N2O4 |
| Molar mass | 370.449 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Development
Samidorphan has been investigated for the treatment of alcoholism and cocaine addiction by its developer, Alkermes,[4][5] showing similar efficacy to naltrexone but possibly with reduced side effects.
However, it has attracted much more attention as part of the combination product ALKS-5461 (buprenorphine/samidorphan), where samidorphan is combined with the mixed MOR weak partial agonist and κ-opioid receptor (KOR) antagonist buprenorphine, as an antidepressant. Buprenorphine has shown antidepressant effects in some human studies, thought to be because of its antagonist effects at the KOR, but has not been further developed for this application because of its MOR agonist effects and consequent abuse potential. By combining buprenorphine with samidorphan to block the MOR agonist effects, the combination acts more like a selective KOR antagonist, and produces only antidepressant effects, without typical MOR effects such as euphoria or substance dependence being evident.[6][7]
Samidorphan is also being studied in combination with olanzapine, as ALKS-3831 (olanzapine/samidorphan), for use in schizophrenia.[8] A Phase 3 study found that the addition of samidorphan to olanzapine significantly reduced weight gain compared to olanzapine alone.[9] The combination is now under review for approval by the US Food and Drug Administration.[10]
Pharmacology
Pharmacodynamics
The known activity profile of samidorphan at the opioid receptors is as follows:[11][12]
- μ-Opioid receptor (Ki = 0.052 nM; EC50 = N/A; Emax = 3.8%; IC50 = 0.88 nM; Imax = 92%)
- κ-Opioid receptor (Ki = 0.23 nM; EC50 = 3.3 nM; Emax = 36%; IC50 = 38 nM; Imax = 57%)
- δ-Opioid receptor (Ki = 2.6 nM; EC50 = 1.5 nM; Emax = 35%; IC50 = 6.9 nM; Imax = 56%)
As such, samidorphan is primarily an antagonist, or extremely weak partial agonist of the MOR.[11][12] In accordance with its in vitro profile, samidorphan has been observed to produce some side effects that are potentially consistent with activation of the KOR such as somnolence, sedation, dizziness, and hallucinations in some patients in clinical trials at the doses tested.[13]
See also
- Tianeptine, an atypical, selective MOR full-agonist licensed for major depression since 1989.
- 8-Carboxamidocyclazocine
- Cyprodime
- JDTic
References
- Turncliff R, DiPetrillo L, Silverman B, Ehrich E (February 2015). "Single- and multiple-dose pharmacokinetics of samidorphan, a novel opioid antagonist, in healthy volunteers". Clinical Therapeutics. 37 (2): 338–48. doi:10.1016/j.clinthera.2014.10.001. PMID 25456560.
- Wentland MP, Lu Q, Lou R, Bu Y, Knapp BI, Bidlack, JM (April 2005). "Synthesis and opioid receptor binding properties of a highly potent 4-hydroxy analogue of naltrexone". Bioorganic & Medicinal Chemistry Letters. 15 (8): 2107–10. doi:10.1016/j.bmcl.2005.02.032. PMID 15808478.
- "Samidorphan". Adis Insight. Springer Nature Switzerland AG.
- Hillemacher T, Heberlein A, Muschler MA, Bleich S, Frieling H (August 2011). "Opioid modulators for alcohol dependence". Expert Opinion on Investigational Drugs. 20 (8): 1073–86. doi:10.1517/13543784.2011.592139. PMID 21651459.
- Clinical trial number NCT01366001 for "ALK33BUP-101: Safety and Pharmacodynamic Effects of ALKS 33-BUP Administered Alone and When Co-administered With Cocaine" at ClinicalTrials.gov
- "ALKS 5461 drug found to reduce depressive symptoms in Phase 1/2 study".
- "Investigational ALKS 5461 Channels 'Opium Cure' for Depression".
- LaMattina J (15 January 2013). "Will Alkermes' Antipsychotic ALKS-3831 Become Another Tredaptive?". Forbes.
- Correll, Christoph U.; Newcomer, John W.; Silverman, Bernard; DiPetrillo, Lauren; Graham, Christine; Jiang, Ying; Du, Yangchun; Simmons, Adam; Hopkinson, Craig; McDonnell, David; Kahn, René S. (2020-08-14). "Effects of Olanzapine Combined With Samidorphan on Weight Gain in Schizophrenia: A 24-Week Phase 3 Study". American Journal of Psychiatry. 177 (12): 1168–1178. doi:10.1176/appi.ajp.2020.19121279. ISSN 0002-953X.
- "FDA Panel: Some Risk OK for Olanzapine Combo With Less Weight Gain". www.medpagetoday.com. 2020-10-09. Retrieved 2021-01-23.
- Linda P. Dwoskin (29 January 2014). Emerging Targets & Therapeutics in the Treatment of Psychostimulant Abuse. Elsevier Science. pp. 398–399, 402–403. ISBN 978-0-12-420177-4.
- Wentland MP, Lou R, Lu Q, Bu Y, Denhardt C, Jin J, et al. (April 2009). "Syntheses of novel high affinity ligands for opioid receptors". Bioorganic & Medicinal Chemistry Letters. 19 (8): 2289–94. doi:10.1016/j.bmcl.2009.02.078. PMC 2791460. PMID 19282177.
- McElroy SL, Guerdjikova AI, Blom TJ, Crow SJ, Memisoglu A, Silverman BL, Ehrich EW (April 2013). "A placebo-controlled pilot study of the novel opioid receptor antagonist ALKS-33 in binge eating disorder". The International Journal of Eating Disorders. 46 (3): 239–45. doi:10.1002/eat.22114. PMID 23381803.